2025 AIChE Annual Meeting
(181p) Biological Production of Xylitol Via Fermentation, Ion-Exchange Separation, and Crystallization
Authors
Sarvada Chipkar - Presenter, Michigan Technological University
Luke Baxter, Boston College
Melanie Cotta, Boston College
Isabella Doyle, Boston College
Gillian Mohr, Boston College
William Rice, Boston College
Claire Richter, Boston College
Charlotte Tonelli, Boston College
Emma Brace, Purdue University
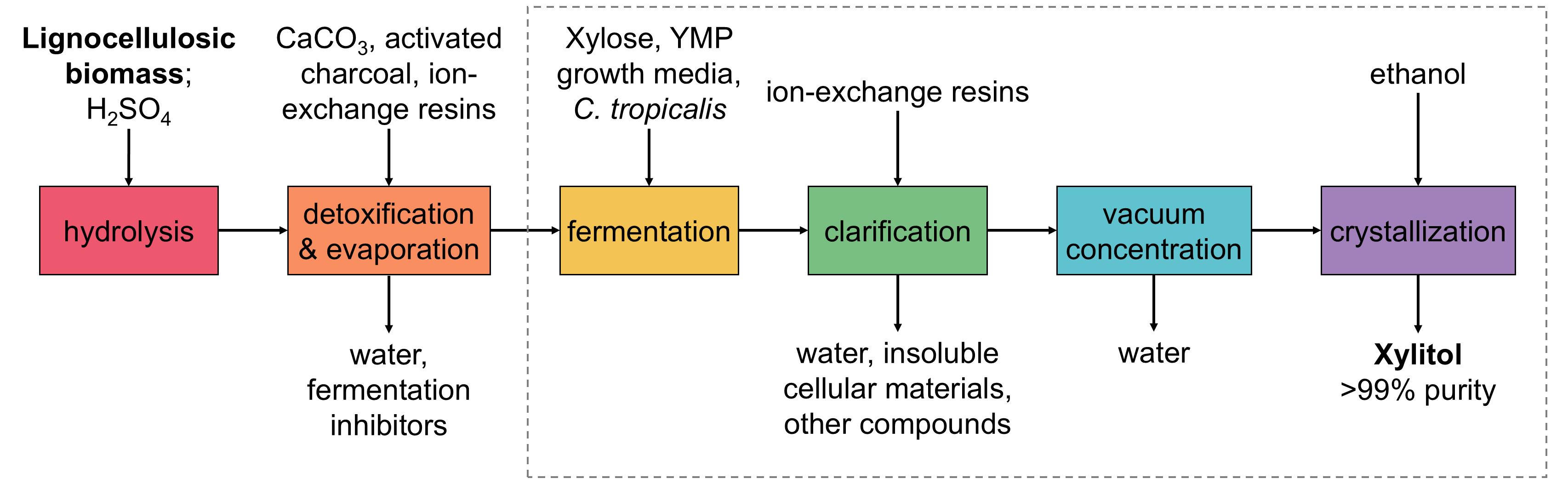
Xylitol, a natural sugar alcohol with commercial applications in both the food and pharmaceutical industries, is one of the platform molecules identified by the Department of Energy as a target for sustainable production from lignocellulosic biomass. While most xylitol is currently produced via thermochemical processes, a biological pathway for xylitol production could present a more cost effective and environmentally friendly solution. The objective of this work is to optimize the fermentation of xylose to produce xylitol, and to subsequently develop new methods for the downstream liquid-liquid separation and purification of xylitol using ion-exchange and crystallization. Using a 10 g/L feed concentration, xylose was fermented using Candida tropicalis with and without pH control using NaOH (this built upon previous work using different xylose feed concentrations and alternate methods of pH control). OD600 spectrophotometry was used during the fermentation to characterize cell growth rates. To separate the xylitol from other analytes in the fermentation broth, the hydrolysate was passed through cationic ion-exchange resin Amberlite IRC-748 using a gravity column. Xylose and xylitol concentrations were then measured using high performance liquid chromatography (HPLC). Vacuum evaporation was used to create a supersaturated xylitol solution, which was then thoroughly mixed with ethanol, and centrifuged to precipitate out the xylitol crystals. The resulting xylitol purity degree was evaluated using a formula derived from previous research. Based on these experiments, further research efforts will include techno-economic analysis and a life cycle assessment of a xylitol biorefinery to evaluate the economic feasibility and environmental sustainability of biological xylitol production.