2025 AIChE Annual Meeting
(483f) Biocompatibility Evaluation of Engineered Albumin-Based Therapeutics in a Murine Model for Potential Treatment of Retinal Degenerations
Authors
Ocular diseases, both chronic and acute, often lead to intricate visual impairments and vision loss. Conditions that cause vision loss, primarily retinal degenerations, share common pathology including oxidative stress and retinal inflammation which result in the accumulation of reactive oxygen species (ROS) and gradual atrophy of the blood-retinal-barrier (BRB). This compromises the immune privilege and barrier function protecting the inner retinal space, allowing cytotoxic metabolites to permeate the retina. These cytotoxic components disrupt visual signal processing and increase the risk of permanent vision loss. Existing therapies are limited by short drug half-lives, the need for frequent and invasive ocular injections, and high healthcare costs. With these treatment obstacles, there is a clinical need for improved therapeutic agents and drug delivery systems to the eye.
This study investigates a novel anti-inflammatory heme-bound human serum albumin (heme-albumin) complex designed to induce heme oxygenase-1 (HO-1) expression in retinal cells. Functionally, HO-1 catabolizes heme degradation into antioxidant and anti-inflammatory byproducts that show the potential for therapeutic benefit. To further enhance ROS scavenging and ensure sustained drug release, the heme-albumin complex is encapsulated in polydopamine nanoparticles (PDA NPs). This study independently evaluates the biocompatibility of heme-albumin and its sustained release system, PDA NPs, in an in vivo mouse model, with a focus on establishing safe dosing for therapeutic efficacy.
Methods
The synthesis of heme-albumin was carried out under basic conditions, followed by purification via tangential flow filtration to remove unbound heme. Protein concentration and heme incorporation were quantified using colorimetric assays. The purity and size distribution of the protein complex were further validated by chromatography in combination with hydrodynamic size analysis. To confirm heme-bound albumin retained its native folding post-synthesis and purification, spectrophotometric analysis was conducted. Additionally, zeta potential measurements were performed to assess surface charge, a key factor influencing protein stability and interactions within biological systems.
PDA NPs were synthesized under basic initiation conditions and purified by centrifugation. NP morphology was assessed through electron microscopy, and their properties as a drug delivery system were further investigated through hydrodynamic sizing and surface charge analysis.
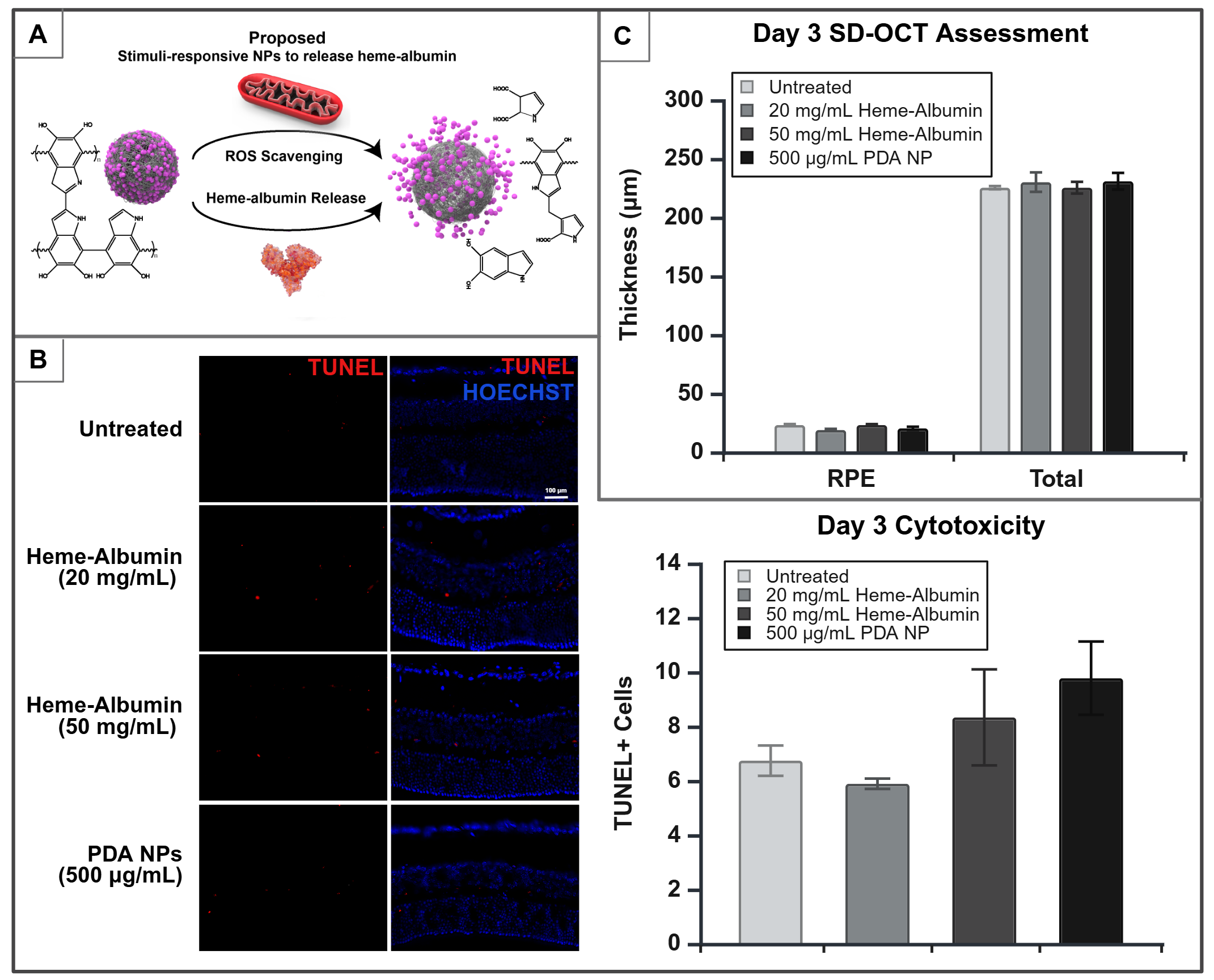
Following the synthesis and characterization of heme-albumin and PDA NPs, adult C57BL/6J mice (8-12 weeks old) were anesthetized and intravitreally (IVT) injected with either heme-albumin (40 and 100 µg) or PDA NPs (1 µg) at a volume of 2 µL. Mice were sacrificed on days 1, 3, and 7 (D1, D3, D7) post-injection and their eyes were enucleated. Ocular tissue was embedded for immunohistochemistry to quantify DNA fragmentation in response to therapeutic doses via TUNEL staining, while HO-1 expression was measured via immunoassay using pooled retinal tissue. In vivo tolerability was evaluated by measuring changes in retinal layer thickness, which were obtained through non-invasive cross-sectional imaging of the retina using Spectral Domain Optical Coherence Tomography (SD-OCT).
Results
Heme-albumin synthesis and purification yielded a stable protein complex, with the secondary structure of heme-bound albumin preserved, as indicated by comparable molar ellipticity between native albumin and the heme-bound form. Size exclusion chromatographic analysis confirmed successful heme incorporation, with a slight increase in molecular weight and a leftward shift in the elution profile of heme-albumin compared to native albumin.
PDA NPs maintained their spherical morphology before and after protein loading, as observed via electron microscopy. Hydrodynamic size analysis showed an increase in particle size upon heme-albumin incorporation, with corresponding changes in surface charge, confirming successful protein loading. These results were further validated by hydrodynamic diameter measurements, which exhibited a Gaussian size distribution for both unloaded and loaded NPs.
Pilot data analysis showed that mice injected with 40 µg of heme-albumin maintained retinal pigment epithelium (RPE) and total retinal thickness at D3 without cytotoxic effects. Increasing the protein complex dose to 100 µg induced HO-1 expression by D1, without compromising retinal structure or causing measurable cytotoxicity on D1 or D3. Additionally, mice treated with 1 µg of PDA NPs exhibited no cytotoxicity at D1, D3, or D7, with intact RPE and total retinal thickness, demonstrating the stability of PDA NPs as a non-toxic, sustained-release carrier.
Conclusion
This study evaluated the biocompatibility and dosing of a novel heme-albumin complex and its delivery system, PDA NPs, for the treatment of ocular diseases. In an in vivo mouse model, varying doses of heme-albumin and PDA NPs were explored to establish therapeutic safety. Biocompatibility assessments confirmed non-toxic dosing ranges. High-dose heme-albumin induced HO-1 expression in retinal cells without compromising retinal structure or inducing cytotoxicity. These findings support the potential of heme-albumin and PDA NPs as redox-responsive agents capable of mitigating oxidative damage and inflammation in ocular diseases. This NP-loaded protein complex will be further evaluated in diseased mouse models to assess its efficacy in treating retinal degeneration and additional ocular conditions.
Figure 1. A. Proposed redox-responsive schematic of heme-albumin loaded PDA NPs. B. Immunohistochemistry micrographs of D3 mice showing TUNEL (red), indicating fragmented DNA, and HOESCHST (blue), staining all DNA. No measurable differences were observed in TUNEL+ cells between untreated, heme-albumin, and PDA NPs-treated mice eyes. C. Retinal layer thickness assessment via SD-OCT in D3 mice. No significant differences were found in the RPE and total retinal thickness between untreated, heme-albumin, and PDA NPs-treated mice eyes.