2025 AIChE Annual Meeting
(398a) Benchmarking COSMO-RS and COSMO-SAC Models for Solubility Prediction of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Author
Experimental solubility data were obtained from the IUPAC-NIST Solubility Data Series[5]. These solubilities were transformed into activity coefficients using the thermodynamic relation involving the Gibbs free energy of fusion, calculated from the experimental melting temperature and enthalpy of fusion for each NSAIDs. This transformation avoids the need for iterative solubility calculations, thereby significantly enhancing the computational efficiency of large-scale model benchmarking.
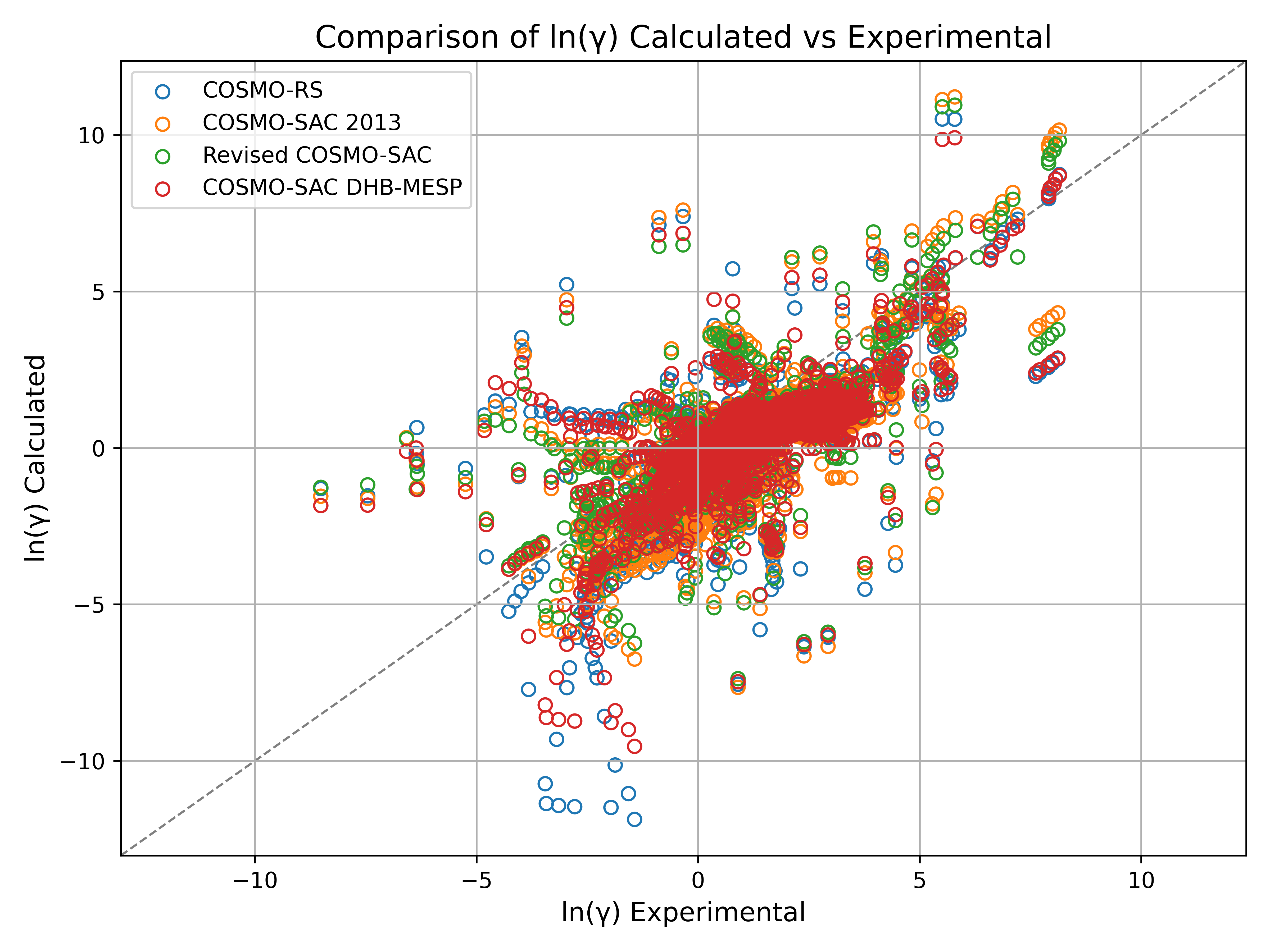
Model accuracy was quantified using the root-mean-square logarithmic deviation (RMSLD) of the activity coefficients, evaluated across 708 data points. The resulting RMSLD values were: COSMO-RS (0.793), COSMO-SAC 2013 (0.806), Revised COSMO-SAC (0.663), and COSMO-SAC DHB-MESP (0.628). These results demonstrate that the COSMO-SAC DHB-MESP method provides improved accuracy compared to the earlier COSMO-RS and COSMO-SAC models.
This benchmark provides a rigorous evaluation of modern COSMO-RS and COSMO-SAC models and highlights the importance of advanced hydrogen bonding descriptions and parameterization strategies for accurate solubility prediction of drug-like molecules in diverse solvents.
Reference
[1] Pye, C. C.; Ziegler, T.; van Lenthe, E.; Louwen, J. N. An Implementation of the Conductor-like Screening Model of Solvation within the Amsterdam Density Functional Package — Part II. COSMO for Real Solvents. Can. J. Chem. 2009, 87 (7), 790–797. https://doi.org/10.1139/V09-008.
[2] Xiong, R.; Sandler, S. I.; Burnett, R. I. An Improvement to COSMO-SAC for Predicting Thermodynamic Properties. Ind. Eng. Chem. Res. 2014, 53 (19), 8265–8278. https://doi.org/10.1021/ie404410v.
[3] Hsieh, C.-M.; Sandler, S. I.; Lin, S.-T. Improvements of COSMO-SAC for Vapor–Liquid and Liquid–Liquid Equilibrium Predictions. Fluid Phase Equilibria 2010, 297 (1), 90–97. https://doi.org/10.1016/j.fluid.2010.06.011.
[4] Chang, C.-K.; Chen, W.-L.; Wu, D. T.; Lin, S.-T. Improved Directional Hydrogen Bonding Interactions for the Prediction of Activity Coefficients with COSMO-SAC. Ind. Eng. Chem. Res. 2018, 57 (32), 11229–11238. https://doi.org/10.1021/acs.iecr.8b02493.
[5] Acree, W. E., Jr. IUPAC-NIST Solubility Data Series. 102. Solubility of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Neat Organic Solvents and Organic Solvent Mixtures. Journal of Physical and Chemical Reference Data 2014, 43 (2), 023102. https://doi.org/10.1063/1.4869683.