2025 AIChE Annual Meeting
(634f) Backpack-Adhered Natural Killer Cell Immunotherapy for Glioblastoma Treatment
Methods. We have adapted our previously established protocol2 with soft lithography, spin-coating, and microcontact printing to develop poly(lactide-co-glycolic) acid (PLGA) microparticle backpacks that are 8 μm in diameter. We have chemically modified the surface of these backpacks to conjugate NK receptor targeting antibodies and then attached the backpacks to the human NK-92 cell line to generate backpack-carrying NK cells. We further characterized the backpack-carrying NK-92 cells by quantifying backpack binding to NK-92 cells with flow cytometry and confocal microscopy. The human GBM cell line U-251 and patient-derived GSC line BT89 will be used for all experiments, including single cells and tumor spheroids to mimic 3D in vivo tumors and cell-cell interactions in the TME. Experimental systems such as brain-mimicking collagen hydrogels, Transwell assays, and microfluidic platforms will investigate the NK-backpack interactions with GBM cells, including under altered IFP through the generation of pressure gradients to generate interstitial fluid flow in the platforms. Confocal microscopy and flow cytometry will be utilized for molecular and biophysical profiling to quantify GBM responses to backpack-based therapy, including cytotoxicity, migration, morphology, cellular phenotypes, and extracellular matrix remodeling.
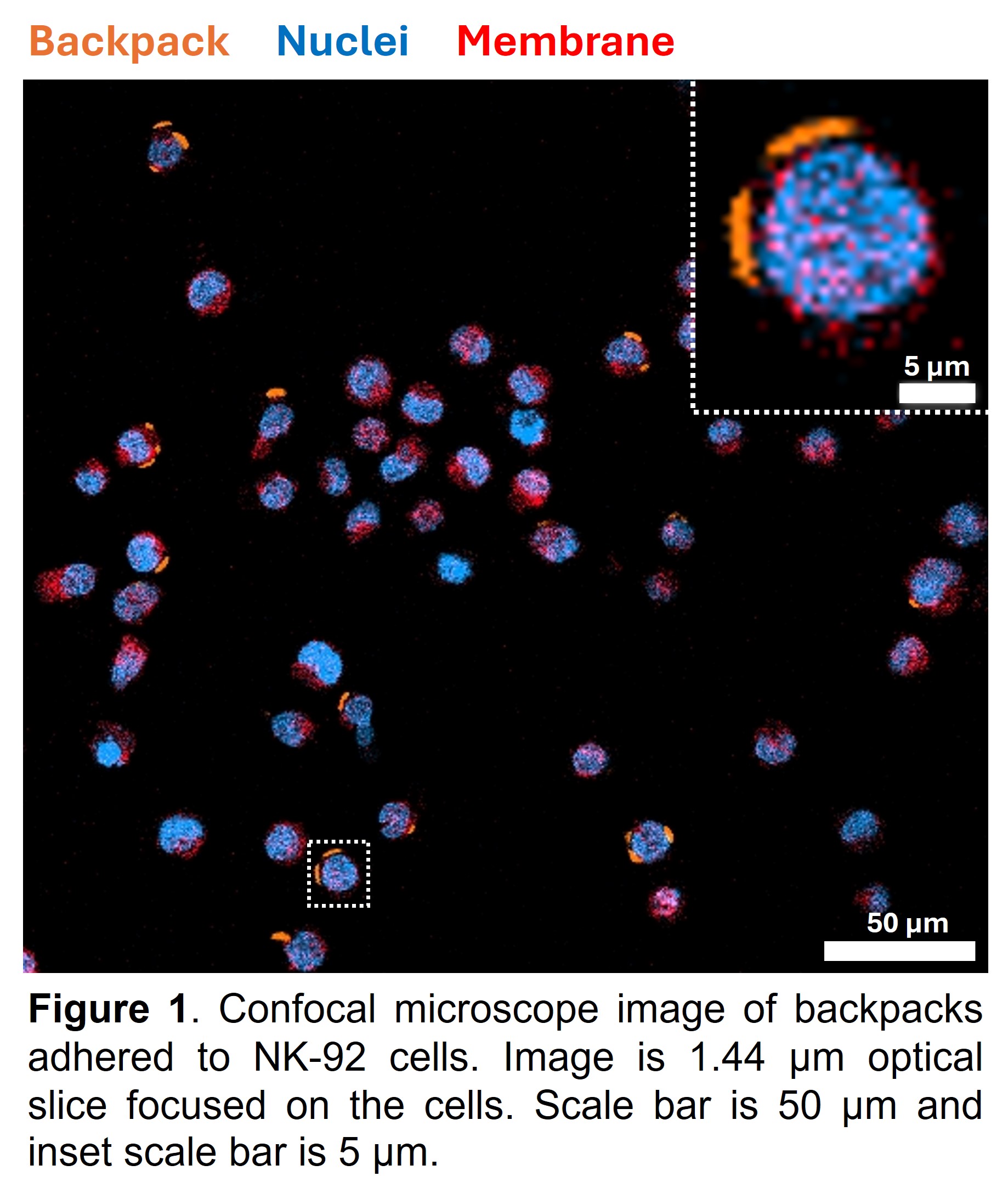
Results, Discussions, and Conclusions: We have demonstrated that our backpack micropatches adhere to NK-92 cells, as illustrated by the confocal microscope image in Figure 1. Furthermore, flow cytometry results have quantitatively confirmed that the backpack synthesis process leads to high backpack binding to NK-92 cells, with 99%-100% of the cells carrying backpacks. Successful completion of this study will be the first step toward developing backpack-mediated NK cell immunotherapy against GBM tumors. Cellular backpack technology is antigen independent, possesses superior tumor infiltration capabilities, and remains effective even in an immunosuppressive TME.2-5 Furthermore, NK backpacks have the potential to target and eradicate tumorigenic, chemoradiotherapy-resistant GSCs, since NK cells can selectively target GSCs and differentiate GSCs to increase their susceptibility to chemotherapy. Cellular backpack-based GBM immunotherapy can thus address issues of tumor heterogeneity to improve patient outcomes. Microfluidic platforms generate gradients in oxygen, nutrients, chemokines, cytokines, and metabolites and enable the integration of TME biochemical and biophysical signals to better recapitulate TME heterogeneity. Our studies will enable an investigation into potential GBM immunotherapy resistance under physiologically relevant conditions, and profiling tumors at single-cell resolutions post-treatment can help identify novel biomarkers and therapeutic targets. These results will thus lend novel insights into GBM patient-specific responses to cell-based immunotherapies.
References
1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. Oct 2014;16 Suppl 4(Suppl 4):iv1-63.
2. Prakash S, Kumbhojkar N, Lu A, et al. Polymer Micropatches as Natural Killer Cell Engagers for Tumor Therapy. ACS Nano. 2023/08/22 2023;17(16):15918-15930.
3. Kapate N, Dunne M, Gottlieb AP, et al. Polymer Backpack-Loaded Tissue Infiltrating Monocytes for Treating Cancer. Adv Healthc Mater. Apr 6 2024:e2304144.
4. Kumbhojkar N, Prakash S, Fukuta T, et al. Neutrophils bearing adhesive polymer micropatches as a drug-free cancer immunotherapy. Nature Biomedical Engineering. 2024/05/01 2024;8(5):579-592.
5. Shields CW, Evans MA, Wang LL-W, et al. Cellular backpacks for macrophage immunotherapy. Science Advances. 2020;6(18):eaaz6579.