2025 AIChE Annual Meeting
(92g) Automated Feedback Control of Monoclonal Antibody Glycosylation through Integrated Process Orchestration
Authors
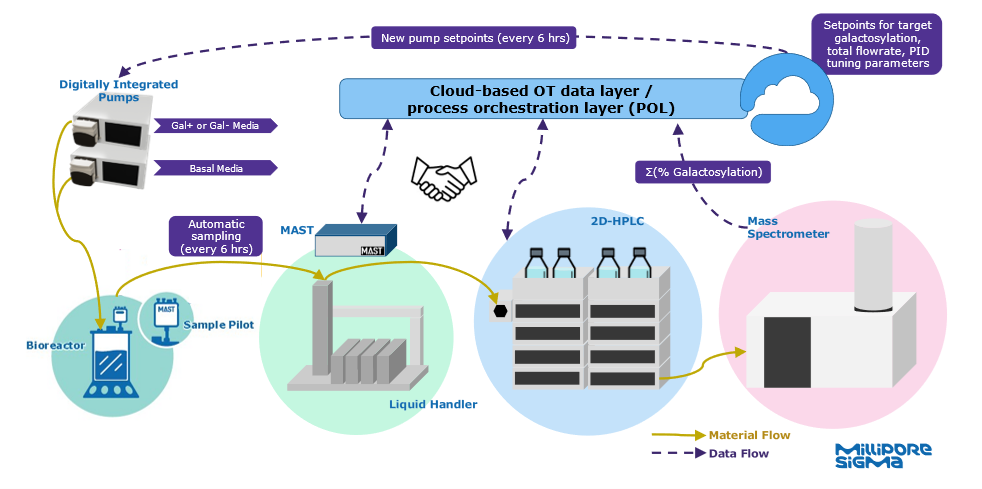
To achieve this, an on-line monitoring system was developed using the MAST® Autosampling Solution and an Agilent 2D-LC/TOF MS system to autonomously monitor the glycosylation on the reduced heavy chain using Flash Characterization to rapidly reduce the antibody. Cellvento® ModiFeed Prime Gal+ and Gal- chemically defined cell culture media was fed into the bioreactor using digitally integrated pumps to modulate mature glycosylation levels. A custom cloud-based process orchestration layer (POL) connected and automated all sampling, LC-MS analytics, and feed media flowrate control. A recipe in the POL allowed for users to enter a desired glycosylation setpoint, modulate sampling and LC-MS analytics frequency, and alter the control method (open-loop or closed-loop). Sample readings were supplied to a control algorithm that updated the media pump flowrates to reach a desired galactosylation percentage (mature glycoform species). The POL also displayed live readings and written progress updates throughout program completion. Real-time data was also displayed in a user-friendly cloud-based dashboard.
Two control schemes were tested: a regression based open-loop control, and a closed-loop PID control. The regression model was generated from bioreactor run data used to correlate certain feed flow rate combinations with specific mature glycosylation levels. Open-loop control updated the pump flowrates based on the desired setpoint and total feed flowrate. If activated, a PID controller trained offline would use the error between recent LC-MS readings and the desired setpoint to update the feed flowrates and minimize the error. With these control scenarios, a total of five galactosylation setpoints were targeted over the course of a 36-day perfusion cell culture process and maintained within a ± 2% deadband around the target setpoint. More than 140 samples were automatically delivered to LC/MS analytics over this period, allowing for efficient data collection and control less than an hour after initial sample draw. The percent sum of unmatured glycoform species, G0 and G0F, were compared to off-line 2-AB glycosylation analysis, with results within practical significance.