2025 AIChE Annual Meeting
(308d) Auto-Oxidation in Redox-Electrodes for the Selective Recovery of Platinum Group Metals and Electrochemical Recycling of Homogeneous Catalysts
Authors
Deborah Schmitt, University of Illinois Urbana Champaign
Johannes Elbert, Massachusetts Institute of Technology
Xiao Su, University of Illinois, Urbana-Champaign
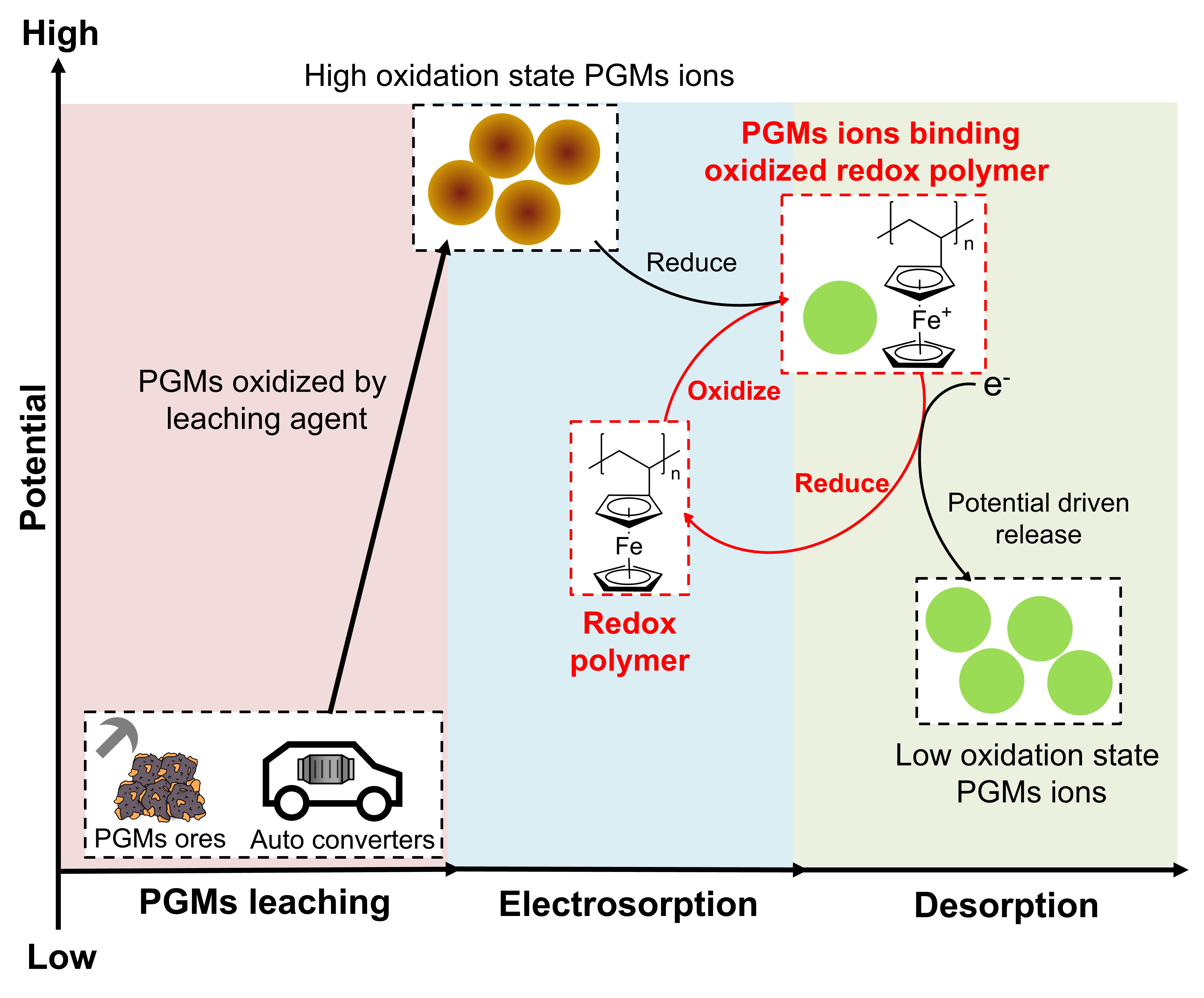
Establishing a circular economy for platinum group metals (PGMs) is essential for the long-term sustainability of the energy industry since their widespread use in automotive catalytic converters, industrial catalysts, and fuel cells. However, due to the close structural similarity between PGMs, the recovery of PGMs requires several separation and purification processes such as precipitation and solvent extractions, which need significant energy and chemical input, and these demanding processes are fraught with pollution and environmental issues. Therefore, platforms with high molecular selectivity and less energy cost are urgently needed. Recently, electrosorption with redox-active materials has been receiving attraction because of the low waste generation and easily tunable target ion interactions.1 Here, we demonstrate an electrochemically reversible selective PGMs recovery system with no external energy input during the adsorption. The system takes advantage of the intrinsic chemical potential owing to the high oxidation state of certain leached PGMs, which forms an auto-oxidation redox couple between redox-functionalized electrodes and PGMs ions, resulting in spontaneous adsorption of PGMs ions. Compared with applying potential for electrosorption, 75% of the energy cost was saved for iridium adsorption with 79% regeneration efficiency.2 Furthermore, the adsorption performance can be enhanced by manipulating the redox properties of the redox materials by introducing different functional groups. These redox materials were applied for recycling industrially relevant homogeneous catalysts used in chemical manufacturing such as Suzuki cross coupling. Results show that the recycled Pd catalysts remained the activity with 91% retention.3 Our work presents a new system for leveraging redox-active materials for energy-efficient PGMs separation. Going forward, we envision the electrochemical recycling system with redox materials to be applied to more realistic industrial conditions such as no electrolyte or non-conductive media via process optimization and polymer design.
References
- Su, X.; Kushima, A.; Halliday, C.; Zhou, J.; Li, J.; Hatton, T. A., Electrochemically-mediated selective capture of heavy metal chromium and arsenic oxyanions from water. Nat. Commun. 2018, 9, 9.
- Chung, C. H.; Cotty, S.; Jeon, J.; Elbert, J.; Su, X., Auto-oxidation of redox electrodes for the selective recovery of platinum group metals. J. Mater. Chem. A 2024, 12 (25), 14.
- Cotty, S.; Jeon, J.; Elbert, J.; Jeyaraj, V. S.; Mironenko, A.; Su, X., Electrochemical recycling of homogeneous catalysts. Sci. Adv. 2022, 8 (42), 12.