2025 AIChE Annual Meeting
(35j) Assessment of Polymeric Materials in CO? Transportation Value Chain: Experimental Characterization and Modeling
Authors
The effective deployment of CCS has to rely on captured CO2 transportation via pipelines or ships, selected based on economic feasibility and operational factors such as distance and emission capacity. These transport modes operate under diverse temperature and pressure conditions, from cryogenic to supercritical states, which influence the performance of polymeric materials [4]. In particular, optimized transport conditions are with dense phases of CO2, i.e. at low temperature or high pressure.
Polymers play a critical role in sealing and protective applications by preventing leakage, mitigating failure, and shielding metal components from corrosive environments. In fact, due to their excellent thermal and chemical resistance thermoplastics can be used as liners, while elastomeric polymers can serve as gaskets and sealants [5]. However, polymers may absorb CO2 molecules that can interact with the matrix by changing the material properties and performances, leading to a plasticizing effect, Glass transition temperature depression or Rapid Gas Decompression (RGD) damage going to affect the stability of the system [6].
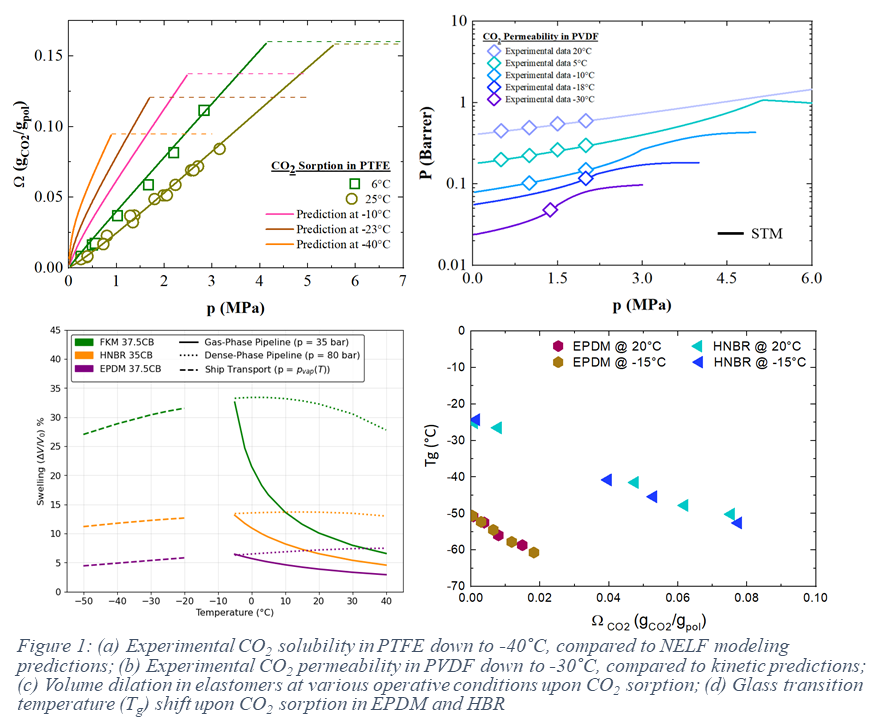
This study presents a comprehensive approach, in which the experimental effort is well complemented by modeling analysis, aiming at the evaluation of the behavior of non-metallic materials, either thermoplastic or elastomers, in contact with dense-phase CO₂. The influence of CO₂ sorption on polymeric materials is examined with an extensive experimental characterization under a wide range of conditions, with focus on the key effects produced, such as swelling and plasticization. Large CO₂ uptakes, indeed, are able to increase chain mobility and free volume, particularly in elastomers, altering mechanical and thermal properties, including reductions in glass transition temperature (Tg), leading to potential issues in structural applications. The barrier effect is also evaluated, exploring in particular the effect of temperature and pressure on gas permeability, as well as the influence of fillers, additives and material properties (e.g., crystallinity).
To elucidate the mechanisms governing CO2 sorption and transport, experimental results are analyzed using thermodynamic equations of state (EoS) for solubility behavior in amorphous polymers, while a standard transport model (STM) describes gas permeation. The proposed tool integrates a thermodynamic model based on EoS to predict penetrant solubility and volume dilation, alongside a kinetic model where gas diffusion is expressed as a function of thermodynamic factors and mobility coefficients. Due to the predictive capability of this approach, a limited experimental dataset is required to estimate CO₂ sorption and swelling across a wide range of conditions. Furthermore, such tool can be conveniently coupled to other approaches able to predict CO2 induced glass transition (e.g. Chow’s model [7]).
The extensive and solid knowledge of thermodynamic and transport properties of CO2 in the different polymers, obtained both from experimental and model analysis, is thus able to provide critical insights into the performance of both thermoplastics and elastomers under gas exposure and the complex interactions between supercritical CO₂ and polymers, supporting the development of resilient materials for the carbon transport chain and future industrial applications.
REFERENCES
- A. Raza, et al., Petroleum 2019, 5, 335, doi:10.1016/j.petlm.2018.12.007.
- J.F.B. Mitchell, Reviews of Geophysics 1989, 27, 115, doi:10.1029/RG027I001P00115.
- U.S. Global Change Research Program Climate Science Special Report: Fourth National Climate Assessment, Volume I. U.S. Global Change Research Program 2018, 1, 470, doi:10.7930/J0J964J6.
- O.M. Davies, et al., J. Mater. Sci. 1999, 34, 417, doi: 10.1023/A:1004442614090.
- M. Nimtz, et al., Geochemistry 2010, 70, 185, doi:10.1016/j.chemer.2010.05.011.
- B. Schrittesser, et al.. Proc. Structur. Integ. 2016, 2, 1746, doi:10.1016/j.prostr.2016.06.220.
- T.S. Chow, Macromolecules, 1980, 13, 362, doi:10.1021/ma60074a029