2025 AIChE Annual Meeting
(450f) Assessment and Optimisation of the Hydrogen and Aluminothermic Reduction Process for the Production of Manganese and Its Alloys
Authors
A novel, low-carbon metallurgical route known as the Hydrogen and Aluminothermic Reduction for Manganese (HAlMan) process has been proposed by Safarian [4] at the Norwegian University of Science and Technology (NTNU). In this process, Mn oxides (primarily MnO2) are first pre-reduced to MnO using hydrogen (e.g. from water electrolysis), releasing H₂O instead of the usual CO₂ from carbothermic reduction. The MnO subsequently undergoes aluminothermic reduction using secondary Al (e.g. scrap or dross) to yield high-purity Mn, Mn alloys, and an Al-rich slag. Emissions of CO2 from the HAlMan process, associated with the decomposition of e.g. calcium and magnesium carbonates in the ore, are negligible compared to carbothermic reduction, assuming green hydrogen and renewable electricity are used.
A modification to the process has also been proposed by NTNU, whereby the Mn ore is pre-calcined, removing moisture, and decomposing the carbonates. This releases a separate stream of CO2, which can be captured and sequestered (or perhaps utilised to make fuels and/or chemicals). This pre-calcination step also has the potential benefit of increasing the ore’s porosity, improving the access of hydrogen to the interiors of the particles; this may increase the rate of pre-reduction, assuming it is not limited by the rate of external mass transfer of hydrogen, decreasing the size of the furnace.
Unlike the conventional carbothermic process, which is highly endothermic, the metallurgical reactions in the HAlMan process are exothermic overall. This offers the opportunity to use excess heat for the generation of electricity, which can be supplemented with renewable energy to produce green hydrogen for the pre-reduction step. Furthermore, HAlMan promotes circularity by extracting Al2O3 from Al-rich slag, from which metallurgical-grade alumina can be produced. Other critical metals, such as strontium and niobium, which are found in trace amounts in the Mn ore, can also be recovered.
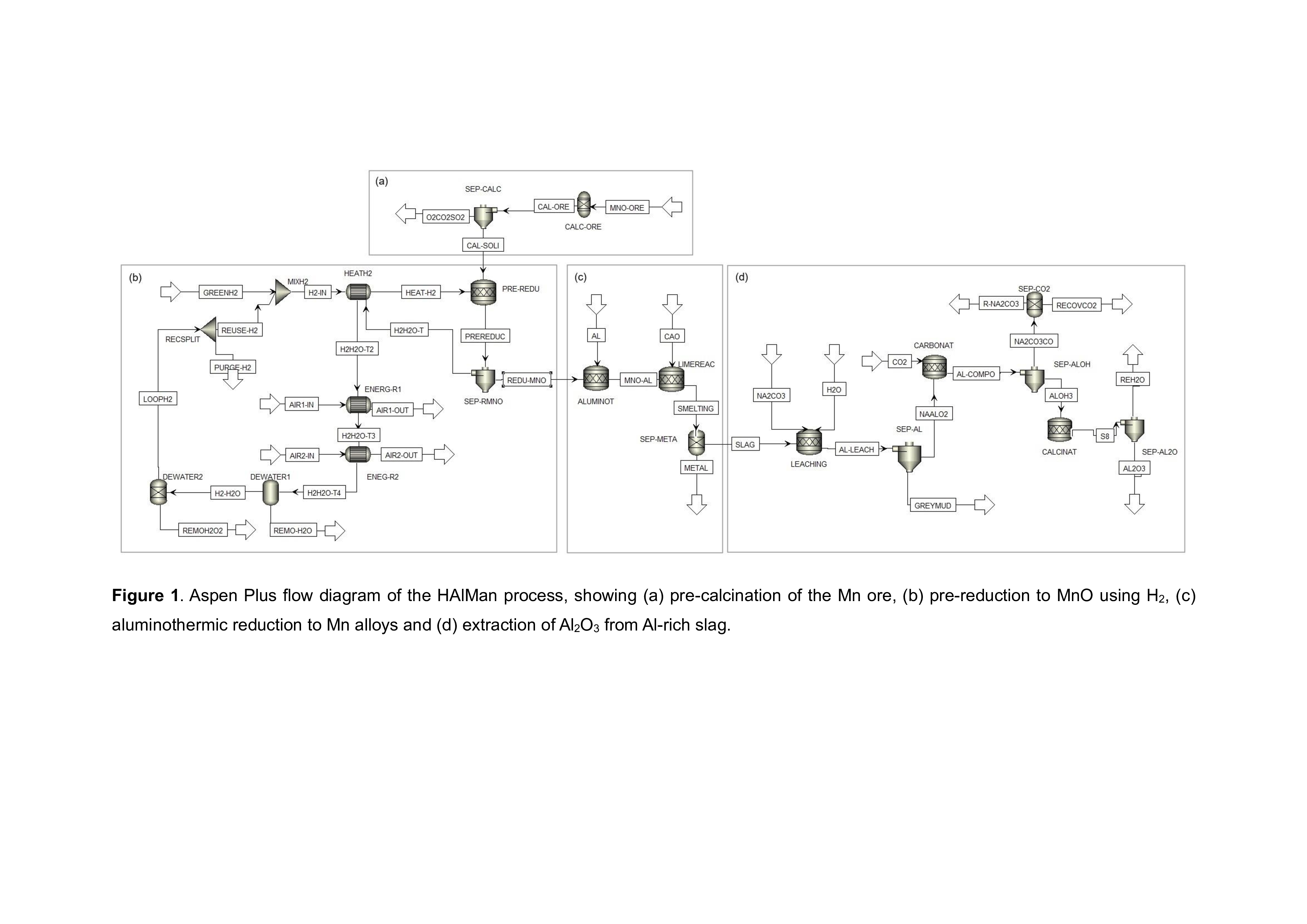
In this study, the HAlMan process is modelled using Aspen Plus, both with and without the pre-calcination step; Fig. 1 shows the flowsheet with pre-calcination. The resulting mass and energy balances are used to assess alternative flowsheets for HAlMan, in terms of energy use and variable operating cost. This approach is used to inform decisions e.g. on whether to recycle excess hydrogen from the reduction step, or to burn it for high-grade heat, and the recovery of excess, lower grade heat to produce electricity. The specific energy consumption of the optimised HAlMan process is compared to that of conventional carbothermic reduction to produce HCFeMn. Initial results indicate that the specific energy input required to produce Mn and its alloys using the HAlMan process is ~ 25% lower than for HCFeMn by carbothermic reduction.
References
[1] U.S. Department of Energy, Critical Materials Assessment, 2023.
[2] European Commission, Study on the critical raw materials for the EU, 2023.
[3] UK Department for Business, Energy & Industrial Strategy, Resilience for the Future: The UK’s Critical Minerals Strategy, 2023.
[4] Safarian, J., A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction. Processes, 2022. 10(1): p. 27.