2025 AIChE Annual Meeting
The Application of Tangential Flow Filtration for the Continuous Processing of Precipitated Monoclonal Antibodies
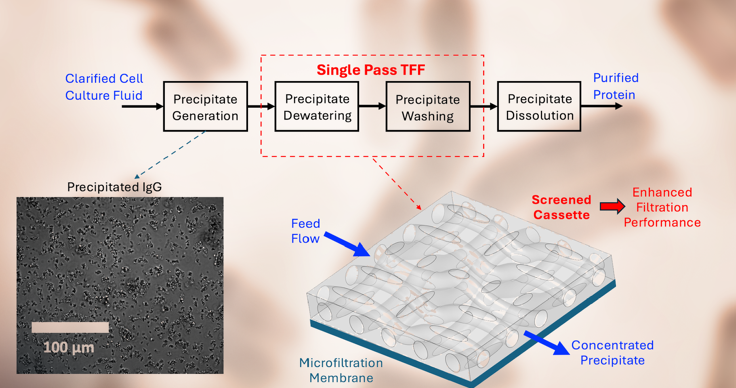
Recent advances in cell culture have led to significant increases in monoclonal antibody (mAb) titer, opening a new window of opportunity for developing a fully continuous downstream purification process for the manufacture of these high-value therapeutic proteins. One approach is to selectively precipitate the mAb from harvested cell culture fluid using ZnCl2 and PEG, with the protein precipitate separated from impurities such as host cell proteins using single-pass tangential flow filtration (SPTFF) with microfiltration membranes. Previous studies have proved that particle morphology has a significant impact on the filterability of the precipitated mAb. In this regard, employing certain salts such as sodium malonate and CaCl2 as densifying agents leads to significantly larger values of the critical flux and maximum conversion in SPTFF. The objective of this work was to examine the potential of obtaining further improvements in SPTFF by using screened membrane cassettes instead of the hollow fiber membranes that have been used in almost all previous studies. The combination of sodium malonate, to the increase in density of the protein precipitate, and the enhanced mass transfer characteristics of the screened cassette resulted in almost a doubling of the critical flux, which was evaluated from well-established flux-stepping experiments. This increase in critical flux provided a large increase in the single pass conversion (ratio of permeate to feed flow rates), enabling much higher concentration factors and enhanced impurity removal compared to that which could be achieved with precipitates formed in the absence of sodium malonate. Further improvements in performance were obtained using two membrane cassettes in series, allowing operation at higher feed flow rates. Data obtained during a continuous 24-hour filtration showed minimal fouling at feed flow rate of 30 ml/min (using two 50 cm2 membrane modules) with over 80% conversion, corresponding to a filtrate flux of 144 LMH. These results demonstrate the potential of using screened membrane cassettes for the continuous / intensified processing of precipitated monoclonal antibodies and other high-value protein products.