2025 AIChE Annual Meeting
(551g) Antibiotic-Loaded Supramolecular Hybrid Hydrogels for Burn Wound Treatment
Authors
SHHs were synthesized with four main components: 1) a water-soluble cationic copolymer (CP) as the guest, 2) cucurbit[7]uril (CB[7]) molecules as the host, 3) exfoliated clay nanosheets (CNSs), and 4) sodium polyacrylate (SPA, anionic). Custom-designed water-soluble acrylamide-random-[3-(methacryloylamino)propyl]trimethylammonium chloride (Am-r-MATMAC) copolymers (CPs) were initially synthesized, allowing the cationic CP guest to interact strongly with the electronegative portals of CB[7] host molecules. CB[7] and the CP were then combined with inorganic clay nanosheets (CNSs) stabilized with sodium polyacrylate (SPA) to enhance the mechanical properties of SHH. Physical characterization, in vitro biocompatibility testing using dermal and epidermal cells, and in vivo biocompatibility evaluation in a mouse model were subsequently performed.
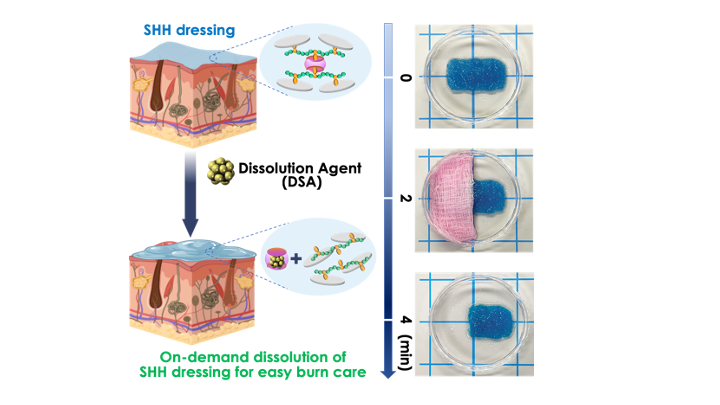
The SHHs formed in 15 seconds, showed high mechanical strength (greater than 50 kPa), self-healed rapidly in 1 min, and dissolved quickly (4-6 min) using amantadine hydrochloride (AH) solution that breaks the supramolecular interactions in the SHHs. Neither the SHHs nor the AH solution had any adverse effects on human dermal fibroblasts or epidermal keratinocytes in vitro. The SHHs also did not elicit any significant immune response in vitro. Furthermore, in vivo murine experiments showed no immune or inflammatory cell infiltration in the subcutaneous tissue and no change in circulatory cytokines compared to sham controls. By incorporating an aminoglycoside antibiotic with broad-spectrum activity, gentamicin, the SHHs effectively prevent bacterial infections. In vitro testing against Staphylococcus aureus and Pseudomonas aeruginosa shows 99% inhibition of bacterial growth inhibition, demonstrating the hydrogel’s potent antibacterial activity. Thus, these SHHs present excellent burn dressing candidates to drastically decrease pain and time associated with dressing changes while preventing bacterial infections.
In summary, we created a simple, quick, and scalable method to synthesize a supramolecular hybrid hydrogel (SHH) via the supramolecular assembly of Am-r-MATMAC CP with CB[7] hosts and CNSs. Our SHH design and synthesis feature green chemistry – whereby we eliminated many toxic and reactive chemicals commonly used in traditional approaches – which also results in rapid hydrogel formation (∼15 s to gelation). As such, these SHHs are the first of their kind as burn dressings, and drastically differ from the existing lengthy and potentially hazardous methods of hydrogel preparation. Due to the ease of their fabrication, we expect these novel SHHs will enable large-scale yet low-cost fabrication, addressing a critical bottleneck for translation to clinical applications. Such novel and on-demand dissoluble SHHs have great potential as second-degree burn dressings. They will i) provide easy burn care, ii) eliminate mechanical and surgical debridement, iii) prevent infection, iv) promote wound healing and enhance the healing process to treat second-degree burns. We expect our invention to remove the challenges and shortcomings associated with current burn dressings and bear pain-free (or reduced pain), easy to apply, and removable burn dressing.