2025 AIChE Annual Meeting
(508e) Advanced Design and Fabrication of 3D-Printed Carbon Monoliths with Tailored Properties and Channel Geometries for Adsorbing Sulfamethoxazole in Water
Authors
Monoliths have proved to be highly advantageous nanostructured porous materials. They are characterized by a single structure composed of thin, vertical, parallel channels separated by walls and a complex macropore network that reduces flow resistance. Due to their unique design and structure, monoliths can effectively mitigate issues related to adsorbent particle arrangement and pressure drops. However, conventional manufacturing methods, such as extrusion, have the disadvantages of restricting the channel geometry to straight channels. Moreover, the porous texture may exhibit imperfections during the manufacturing process, leading to uneven porosity. The absence of uniform porosity and the inability to precisely control channel geometry, confine fluid dynamics to laminar flow within the channels. This effect can hinder the mixing and distribution of the fluid, resulting in minimal adsorbate-adsorbent interaction and the formation of preferential flow paths.
In this sense, a novel hybrid methodology that couples sol-gel polymerization with 3D printing technology is proposed for the manufacturing of precisely ordered 3D-printed carbon monolith adsorbents to remove sulfamethoxazole (SMX) from water, a hazardous antibiotic commonly found in water solutions, in both batch and fixed-bed adsorbers. Sol-gel polymerization allows the controlled synthesis of high-purity nanostructured carbon gels with precise tuning of parameters such as shape, chemical composition, particle size, and pore size distribution. In parallel, 3D printing facilitates the fabrication of highly customized three-dimensional prototypes and structures based on digital designs, enabling the manufacture of complex channel geometries that are impossible to achieve through conventional methods. This innovative method represents a groundbreaking advancement in fixed bed design packing, as no previous studies have successfully integrated these techniques to develop 3D-printed monolithic beds for liquid phase adsorption. This study evaluates the influence of synthesis conditions on the monolith morphological, textural, chemical, and mechanical properties by varying the resorcinol/catalyst ratio. In addition, the impact of synthesis conditions on SMX adsorption isotherms and breakthrough curves is investigated in this work. Finally, the influence of channel geometry is assessed by obtaining the breakthrough curves using a monolith configured with conventional straight channels as a reference. Computational Fluid Dynamics (CFD) calculations further confirm the observed adsorption behavior.
The channel geometries for the 3D-printed monoliths were strategically designed using SolidWorks software and then printed in an Ultimaker S3 3D printer, ensuring high precision and structural detail. Two distinct types of CAD models were fabricated: a 3D model representing a complex network of interconnected channels extending in multiple directions and a more traditional design with linear channels aligned along the axial direction, similar to conventional monoliths manufactured by extrusion.
Solutions comprising resorcinol (R), formaldehyde (F), water (W), and the catalyst Cs2CO3 (Cs) were prepared to synthesize the monoliths, maintaining specific molar ratios: R/F = 1/2 and R/W = 1/15. The porosity of the resulting monoliths was tailored by varying the R/Cs ratio at 100, 500, 1000, and 2000. The solutions and the 3D-printed templates were placed into cylindrical glass molds and hermetically sealed. The polymerization and curing process was completed over 1 day at ambient temperature, followed by 1 day at 50 °C, and concluding with 5 days at 800 °C. Upon completion, the resulting organic polymer was de-molded and sectioned to desired dimensions. The water occupying the developed porosity was then displaced with acetone over 3 days, and the solution was changed twice daily. Afterward, organic monoliths were fabricated with the 3D-printed template integrated into the organic gel matrix.
Prior to carbonization, the organic monoliths were dried at room temperature in a N2 flow of 150 mL/min. The carbonization process involved a preliminary treatment at 300 °C for two hours and a second heating stage at 850 °C for two more hours. In both cases, the heating rate was 1.5 °C/min. During the initial carbonization, the 3D-printed template was removed, forming a complex network of interconnected channels within the resulting carbon monoliths. These monoliths were designated Monolith-100, Monolith-500, Monolith-1000, and Monolith-2000, based on the variation in the R/Cs ratio. The effect of channel geometry in the monolith was evaluated by fabricating a monolith with conventional straight channels using an R/Cs ratio of 1000.
The adsorption capacities of the 3D-printed carbon monoliths for SMX were determined in a batch adsorber with initial concentrations ranging from 25 to 200 mg/L, at T = 25 °C and pH 7, using representative slices of each monolith, cut to a specific mass. The adsorption isotherms of SMX on carbon monoliths with varying R/Cs ratios were interpreted using the Freundlich, Langmuir, and Radke-Prausnitz isotherm models.
Breakthrough curves for SMX adsorption were obtained in an up-flow monolithic packed bed adsorber. The adsorption column was packed with glass beads to a bed height of 3 cm to ensure homogeneous flow distribution of the solution before entering the monolith packing. Subsequently, a 3D-printed carbon monolith was placed into the column, and glass beads were added to fill the acrylic column. The monolith packing in the bed was contacted with a contaminant-free electrolyte solution at pH 7 for 24 h to eliminate entrapped air in the packed bed. After the preconditioning period, an SMX solution (initial concentration, CAo = 50 mg/L) was continuously fed to the packed bed at a specific volumetric flow rate and pH = 7 and T = 25 °C. Effluent samples were collected at various time intervals using the autosampler, and the SMX concentration of these samples was quantified using UV-Vis spectroscopy.
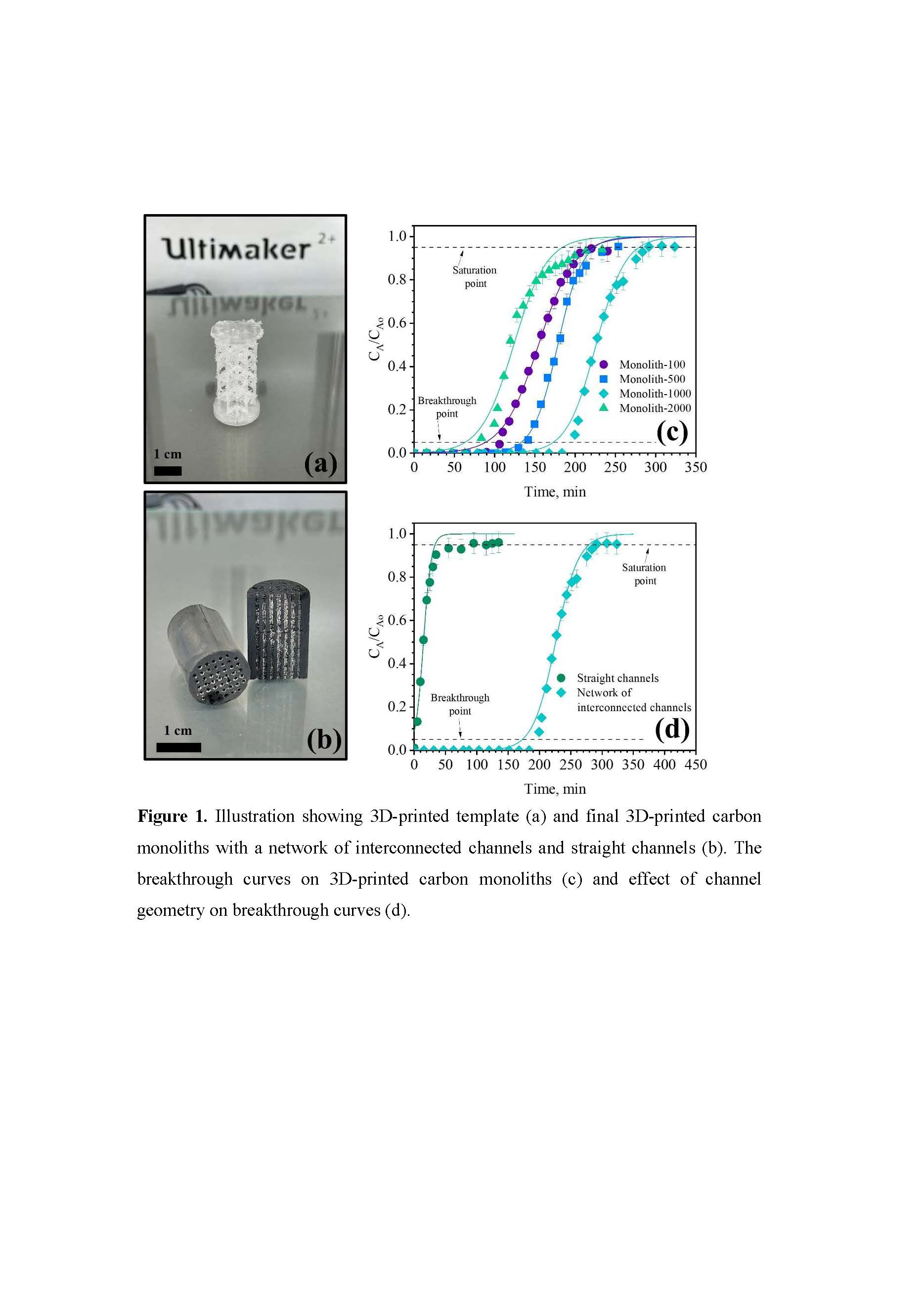
Figure 1(a) shows the designed 3D-printed template exhibiting flawless replication of the original CAD model. The organic monoliths post-polymerization and curing (Figure 1(b)) show that the 3D-printed template seamlessly integrates within the organic gel matrix, with no noticeable gaps in the walls or at the interface between the organic gel and the 3D-printed template. The final 3D-printed carbon monoliths demonstrate the successful template removal after carbonization, resulting in flawlessly conforming network of interconnected channels and straight channels (Figure 1b) within the integral carbon bulk. These results demonstrate the ability of 3D printing technology to perfectly control the configuration of sophisticated channel geometries within the carbon monoliths, highlighting its precision and versatility in fabricating intricate designs.
Increasing the R/Cs ratio resulted in larger spherical primary particles, which modulated mean pore diameter and skeleton size within the macropore region (ranging from 95.1 to 157.1 nm). This adjustment enhanced accessible macroporosity and reduced flow resistance, improving monolith adsorption performance. The adsorption isotherms showed a maximum adsorption capacity of 61.4 mg/g for Monolith-100, attributed to π-π stacking and electrostatic attractions, and the decreasing order of the adsorption capacity was: Monolith-100 > Monolith-500 > Monolith-1000 > Monolith-2000. However, a different trend was observed in dynamic adsorption (Figure 1(c)): Monolith-1000 > Monolith-500 > Monolith-100 > Monolith-2000. Increasing the R/Cs ratio improved SMX accessibility, resulted in longer breakthrough times and reduced mass transfer zone height, despite the reduction in external area. However, an excessively high R/Cs ratio (Monolith-2000) has been found to significantly decreased the overall adsorption performance by reducing breakthrough times.
The channel geometry of the monoliths significantly influenced breakthrough times (Figure 1(d)). The complex network of interconnected channels promoted more intricate flow patterns, enhancing mixing and fluid distribution, and facilitating SMX adsorption throughout the porous structure. In contrast, the conventional straight channels showed a laminar flow pattern, which led to preferential flow paths and limited interaction with the adsorbent material. This detailed fluid dynamic analysis explains the significant differences in breakthrough times and adsorption performance observed experimentally.