2025 AIChE Annual Meeting
(399d) The Adsorption of Carbon Monoxide at High Temperatures
Authors
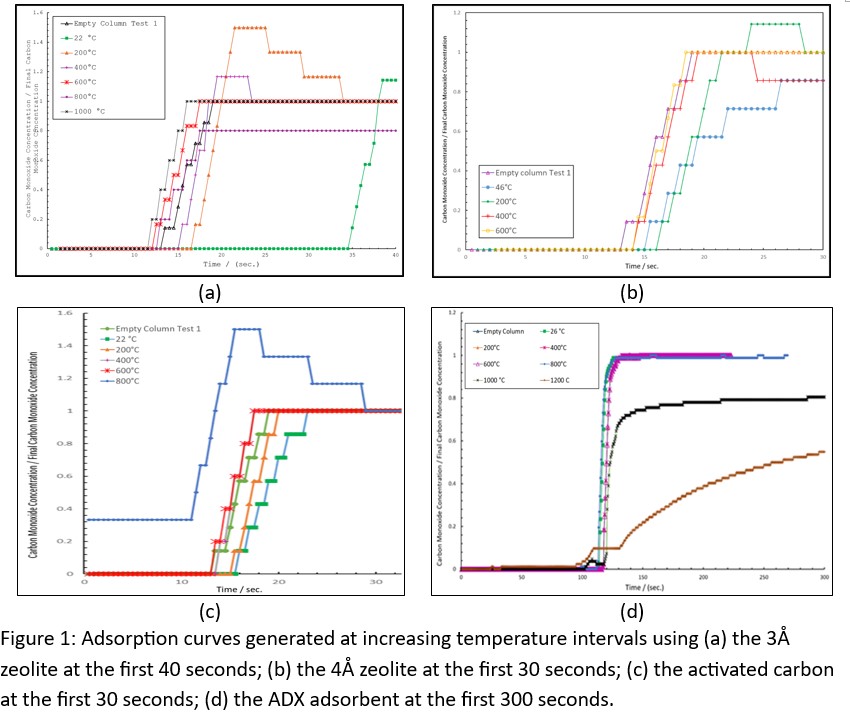
Preliminary CO adsorption tests [2] were performed at varying temperature on an adsorption column using 3Å Zeolite, 4Å Zeolite, activated carbon, and our novel adsorbent, (named ADX after our authors’ name: Arndt-Davis-Xie adsorbent), as shown in Figure 1. The 3Å zeolite was tested up to 1000°C and found to greatly reduce its adsorbing of carbon monoxide at temperatures over 400°C. The 4Å zeolite was tested to 600°C and suffered the same failing as the 3Å zeolite losing its ability to adsorb CO at 400°C. The activated carbon sample was tested up to 800°C. It also suffered a large drop in adsorption at 400°C but didn’t completely fail to adsorb CO until it reached 600°C. As the temperature increased the carbon and zeolite samples’ ability to adsorb CO decreased. The ADX adsorbent has shown itself to be capable of adsorbing CO at temperatures exceeding 1,000°C. Further research needs to be performed to ascertain to what extent ADX adsorbent can be applied at high temperatures.
References:
[1] Ma, Xiaozhou, Jelco Albertsma, Dieke Gabriels, Rens Horst, Sevgi Polat, Casper Snoeks, Freek Kapteijn et al. (2023). "Carbon monoxide separation: past, present and future." Chemical Society Reviews 52, no. 11 (2023): 3741-3777.
[2] Arndt, L.D. (2024) “Adsorption of Carbon Monoxide for Magnesium Production by Carbothermic Reduction,” M.A. thesis, Dept. Chemical Engineering, University of Minnesota, Duluth, MN, USA.