2025 AIChE Annual Meeting
(348e) Acid Catalytic Conversion of High Density Polyethylene to Nylon Precursors
Mineral acid treatments present a practical and sustainable solution towards upcycling recalcitrant plastic wastes such as Polyethylene (PE) since they are cheap, readily available, can act as both solvent and oxidizer, and can be readily regenerated using water and oxygen. Previous literature and patents describe processes converting PE to dicarboxylic acids using nitric acid but tend to form less desirable nitro dicarboxylic acid products or obtain low carbon yields.
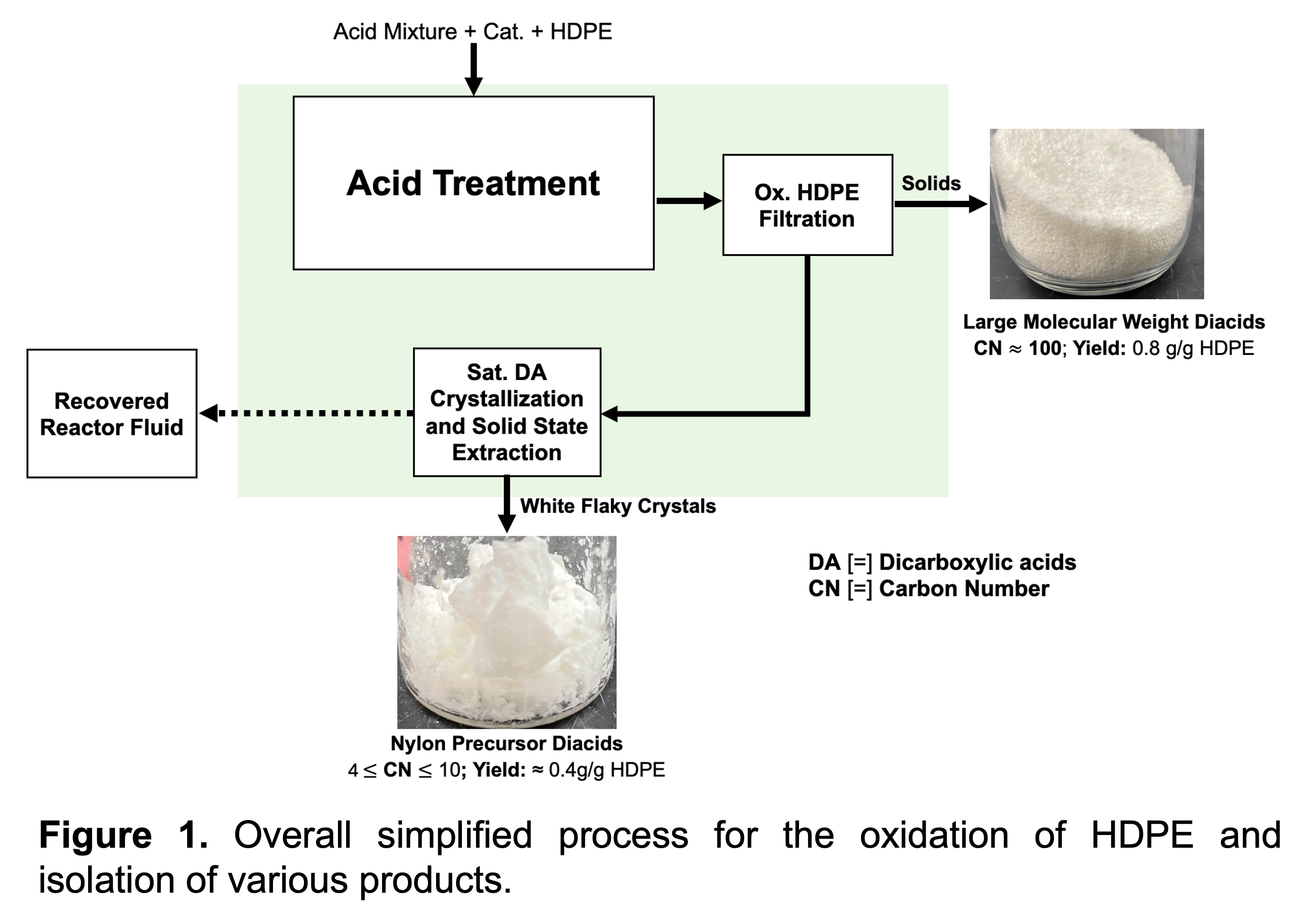
To address this problem, a new one-pot process was developed, which utilizes a novel mixture of acids and inexpensive catalyst at moderate temperatures and ambient pressures to degrade high density polyethylene (HDPE) to aliphatic dicarboxylic acids. Removal of residual functionalities, such as nitro groups, to form pure dicarboxylic acids was found to be the result of the acid mixture and catalyst participating in a catalytic cycle. Adjustments to reaction parameters enabled fine control of the molecular weight distribution of dicarboxylic acid products with carbon numbers (CN) ranging between 4 and 150. Particularly, a moderate yield of valuable Nylon precursors (4 ≤ CN ≤ 10) around 0.4 g/g of HDPE were readily isolated with most of the remaining carbon recovered as large molecular weight acids (CN ≈ 100, 0.8 g/g of HDPE). This approach led to process intensification, such as four-fold fewer unit operations with a comparable reduction in process time and atom economy compared to processes described in the literature. The novel acid-catalyst system for upcycling PE marks a major step towards the necessary development of elegant plastic recycling processes that enable economic recovery of carbon waste as platform chemicals for sustainable material production.