2025 AIChE Annual Meeting
(394ak) Accelerated Reaction Discovery Via High-Throughput Reaction Pooling and Progressive Fidelity Analytics
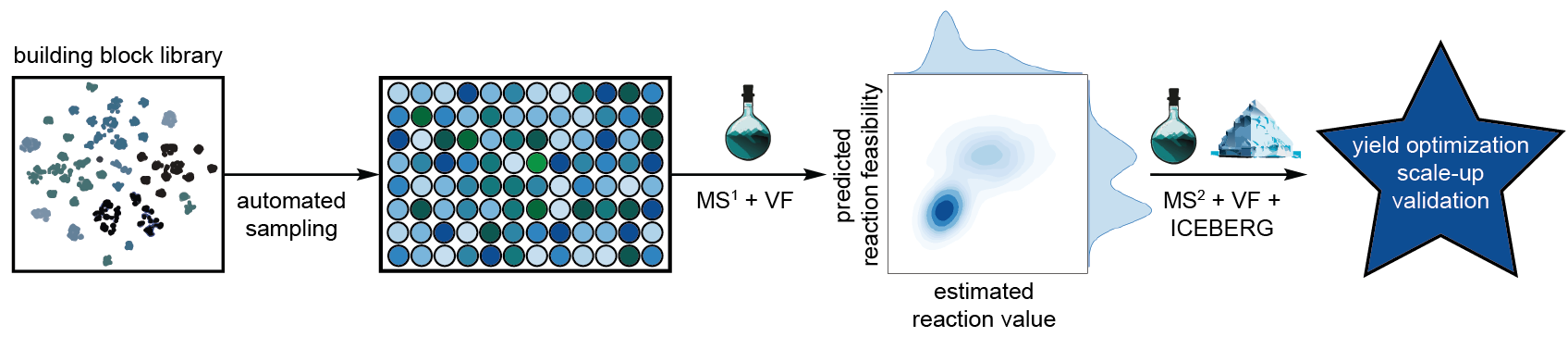
Novel reactivity is essential in optimizing access to valuable and/or novel areas of chemical space. However, the absence of systematic methods to detect and assess novel reactions limits our ability to translate chemical diversity into practical innovation. We have previously shown how modern informatics in conjunction with automation can be used to rapidly hypothesize and evaluate novel multicomponent chemistry for value and feasibility using our virtual flask software. Further advances in automated synthesis, experimental design philosophy, and analytical chemistry techniques have extended this workflow to rapidly maximize the information gain from any experiment by mapping mass spectra to mechanistically enumerated product structures. We report a tiered, end-to-end reaction/molecular discovery pipeline that rapidly identifies promising chemical transformations using a design of experiment strategy that combines high-throughput pooling and fidelity-tiered analytics. Substrate and reagent combinations are sampled from our inhouse inventory and subjected to rapid, low-fidelity screening under standardized conditions using low-resolution, high-throughput UPLC-MS. Potential high value reactions are identified based on predicted molecular structures matching low-resolution mass spectrum peaks, and then downselected for further analysis using a composite score that estimates the value, feasibility, and scalability of the hypothesized reaction. Promising reaction crudes are stamped and batched before being assessed at a higher fidelity using tandem mass spectroscopy, spectral cheminformatics, and machine learning driven structure elucidation models. Finally, reactions confirmed at this confidence are optimized for yield, scaled-up, and isolated for validation by NMR.