2025 AIChE Annual Meeting
(459e) A 3D-Printed Gyroid Ni/Al2O3 Catalyst for CO2 Methanation
Authors
Ayesha Alkhoori, ETH Zurich
Miguel Palomino, Instituto de Tecnologia Quimica (CSIC-UPV), Universidad Politecnica de Valencia
Lorena Maria Andres Olmos, Instituto de Tecnología Química (UPV-CSIC), Universitat Politècnica de València?Consejo Superior de Investigaciones Científicas
Antonio Chica, 3Instituto de Tecnología Química (UPV-CSIC), Universitat Politècnica de València?Consejo Superior de Investigaciones Científicas
Fernando Rey, Instituto de Tecnología Química
Susana Valencia, Instituto de Tecnología Química
Kyriaki Polychronopoulou, Khalifa University of Science and Technology
Rashid Abu Al-Rub, Khalifa University
Nahla Alamoodi, Khalifa University of Science and Technology
Georgios N. Karanikolos, Khalifa University
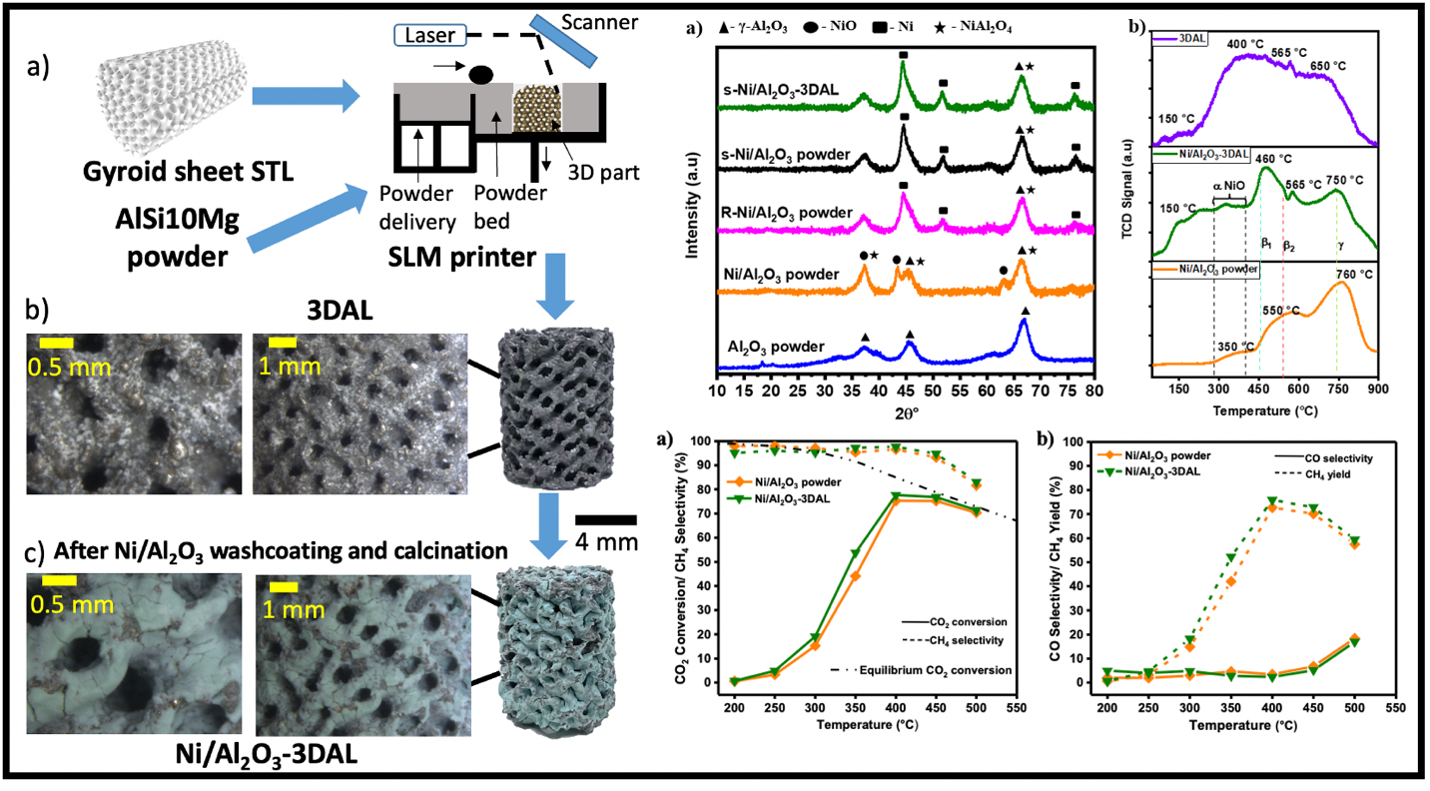
The utilization of 3D-printing in catalyst production for CO2 methanation has emerged as a response to the challenges posed by the highly exothermic reaction and high gas space velocity, conditions that necessitate enhanced heat and mass transfer while maintaining optimal catalytic performance1. In this work, we developed a new CO2 methanation catalyst comprising a Ni/Al2O3 powder-coated 3D-printed aluminum alloy of gyroid configuration. The metallic aluminum alloy (AlMgSi) was 3D-printed (3DAL) using selective laser melting (SLM), and Ni/Al2O3 powder was coated on it by washcoating. Microscopy and tomography techniques were employed to examine the morphological characteristics of the catalyst and to analyze internal topology, and hydrogen temperature-programmed reduction (H2-TPR) and chemisorption provided insights into the reduction sites and active metal phase. The catalytic performance was assessed through CO2 methanation experiments conducted at various temperatures ranging from 250 °C to 500 °C, using a CO2:H2:He gas mixture (1:4:5). The 3D-printed Ni/Al2O3-3DAL catalyst exhibited high CH4 selectivity (97.7%) and CO2 conversion (77.6%) at 400 °C, which can be attributed to the reduced tendency of sintering and the effective heat transfer owing to the metallic support. The 3D-printed gyroid-sheet metallic support provided a higher surface area-to-volume ratio, enabling higher catalyst loading per unit volume and improved reactants contact with the active catalyst phase yielding enhanced catalytic performance compared to powder. It also offers potential for enhanced thermal energy management and mechanical strength compared to conventional beads and pellets.
References:
- Jivrakh KB, Kuppireddy S, Dumée LF, et al. A critical review on 3D-printed adsorbents, membranes, and catalysts for carbon dioxide capture, separation, and conversion. J Clean Prod. 2024;472:143522. doi:10.1016/J.JCLEPRO.2024.143522