2024 AIChE Annual Meeting
Quantitative Analysis of Atomic Resolvability and Radiation Damage in Apoferritin Complexes Using Cryogenic-Electron Microscopy and Q-Score Evaluation
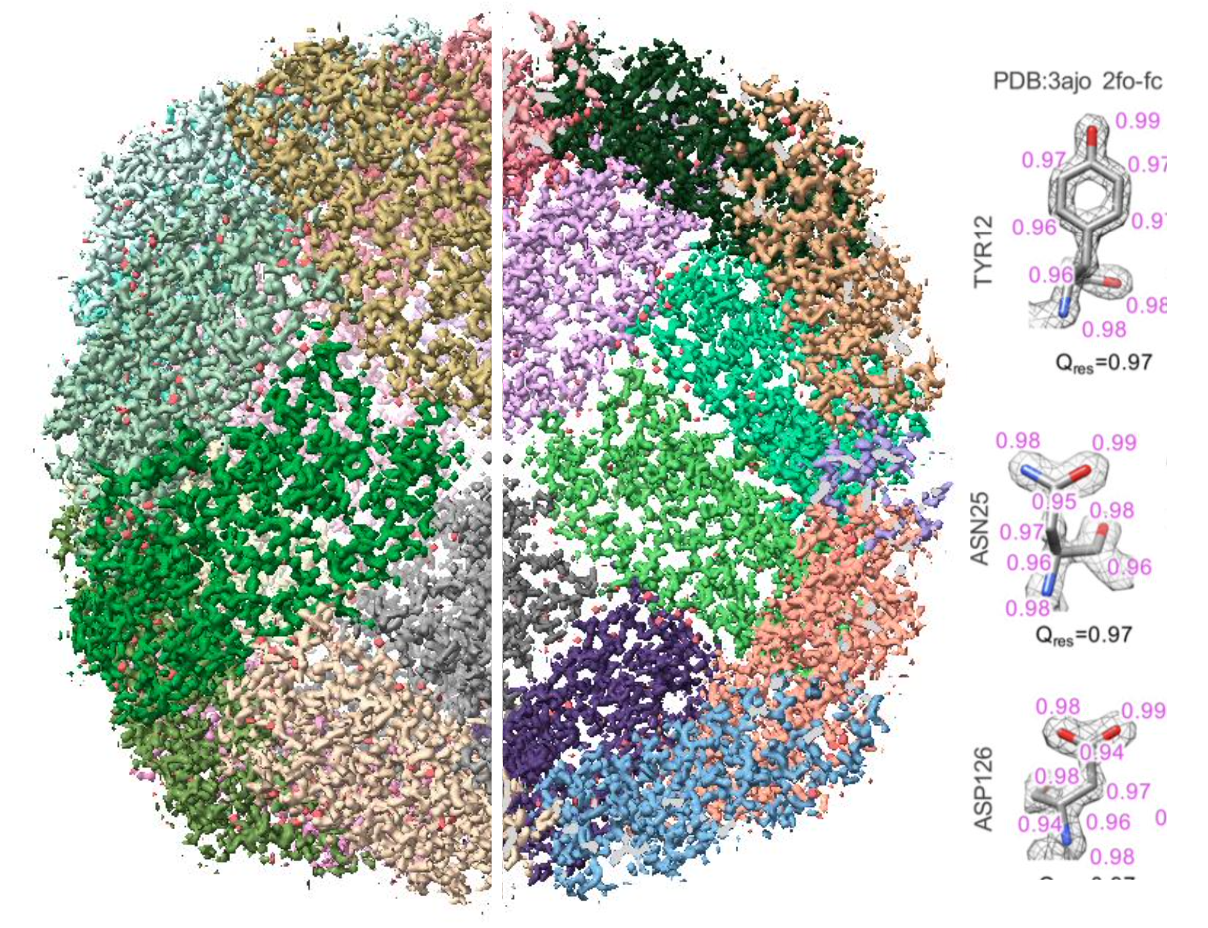
Electron radiation damage is well-known to limit the resolvability in density maps reconstructed from images of single particles in cryogenic-electron microscopy (Cryo-EM). However, it is not clear whether this damage affects all side chains equally. In this study, we analyzed a set of images of vitrified Apoferritin particles that could yield a map with a final resolution of 1.69Å. To assess the effects of radiation damage at the side chain level, we computed the Q-scores of maps and models generated from later sets of frames in the movies corresponding to higher cumulative doses. Our analysis revealed that the map resolution, as computed from the Fourier Shell Correlation (FSC), decreased with increasing cumulative doses of the corresponding maps. Furthermore, the Q-scores (i.e. resolvability) of the side chain densities declined at a faster rate for negatively charged residues compared to positive and non-polar residues. We observed that aromatic residues exhibited greater structural preservation under similar cumulative dose conditions, while in the case of negative charged residues, the density resolvability could arrive from inherent scattering properties. To isolate the scattering effect from the dose effect, we modified the original map by both 1) phase randomization and 2) equalizing amplitudes at resolution lower than 1.69A in two different tests. This approach allowed us to isolate the impact of radiation damage from the scattering effects of negatively charged atoms. The observed declines in Q-score and structural visibility in the maps with higher cumulative doses were indeed attributed both to dose-induced damage and to scattering differences at low resolution, particularly involving negatively charged oxygen atoms. We demonstrated that signal falloff occurs more rapidly with increased dosage, particularly in the critical 2.1 Å and 3.2 Å resolution ranges where protein side chains, ions, and waters undergo radiolytic processes. This suggests that inherent limitations in cryo-EM's structural fidelity may exist, independent of radiation effects, potentially due to the intrinsic scattering properties of the sample or other factors such as side chain dynamic motions.