2024 AIChE Annual Meeting
Optimizing Myoblast Attachment to Shape Memory Biodegradable Elastomers
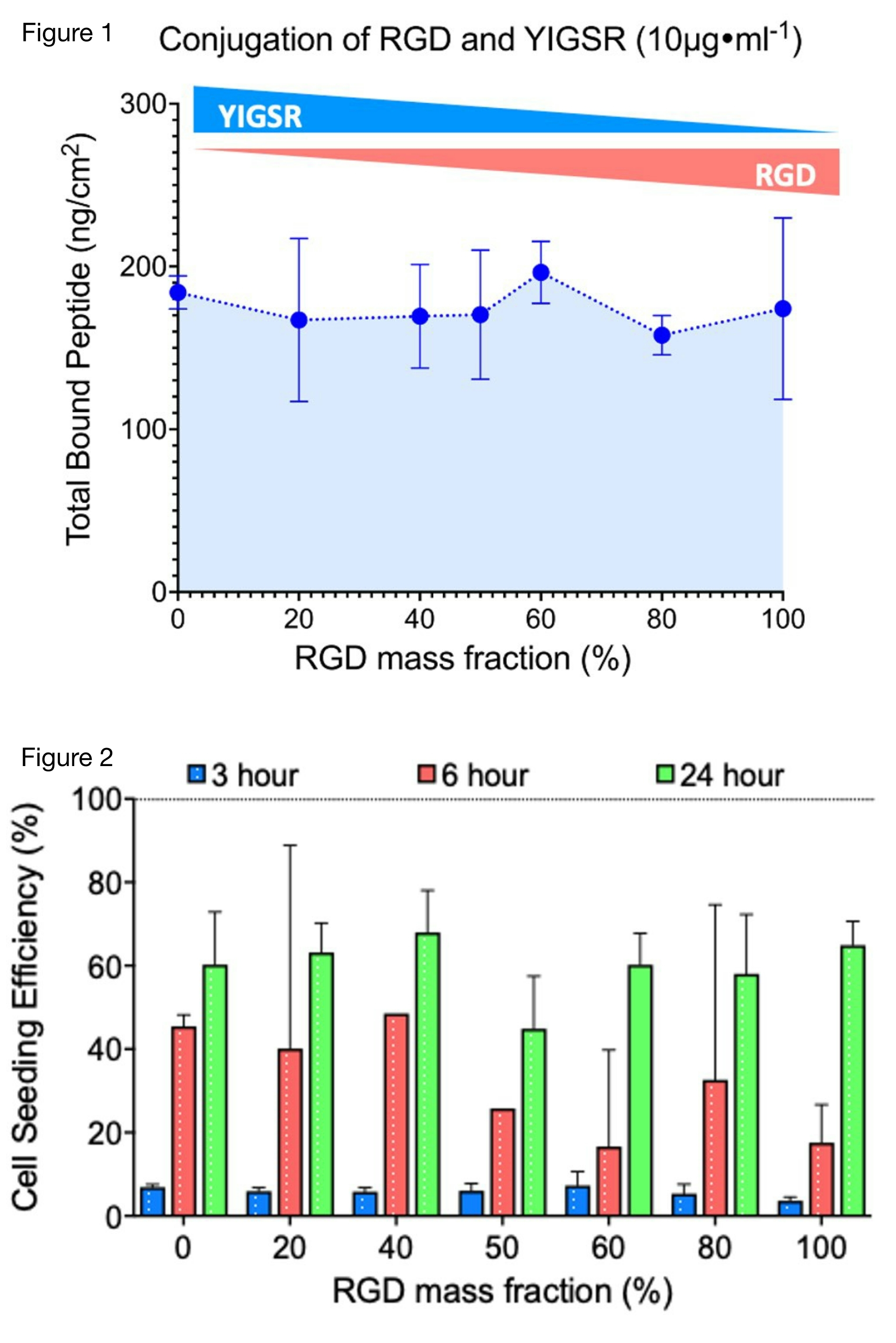
We hypothesized that CAMs increase overall murine myoblast attachment, with higher attachment shown in the laminin binding domain YIGSR dominant mass fractions. To test this, PGD was cast into a 96-well plate, sterilized with ethanol, and functionalized with seven mass fractions of RGD:YIGSR (100%-50%). A CBCQA Assay and One-way ANOVA analysis revealed, uniform peptide binding observed across all mass fractions (Figure 1), establishing a consistent baseline for cell attachment.
Murine myoblasts (C2C12s) were seeded onto the peptide-enhanced substrate in triplicates and quantified via fluorescence intensity and a Calcein assay. Results, analyzed with Two-Way ANOVA, show differences in cell attachment between mass fractions at varying time points. At the 24-hour mark, seeding efficiency reached 60% across all mass fractions (Figure 2), with YIGSR dominant mass fractions (100%-50%) having significantly greater attachment at the 6-hour mark. We perceive that Murine Myoblasts attach better to YIGSR due to prior work that indicates their ability to interact with the extracellular matrix laminin binding integrin receptor α7-β1.
Despite biological variability, these findings suggest the addition of CAMs, specifically YIGSR alongside RGD, enhances cell attachment and reduces attachment time. Future studies will investigate the use of micropatterned peptide-enhanced substrates to further optimize cell attachment and alignment.