Personalized medicine is an important topic in contemporary healthcare, aiming to tailor medical treatment to individual patients. However, 2D modeling platforms are not as physiologically relevant as 3D models because they cannot reproduce relevant aspects of native tissue such as cell-cell interactions, proliferation, the extracellular matrix (ECM), and tissue development. Spheroids are a type of 3D model that offer a superior representation of solid tumor complexities compared to other models. Despite their advantages, spheroid production is limited due to time consumption and reproducibility. Given that treatment decisions are often required within 14 days of diagnosis, there is a pressing need for a more efficient fabrication method to ensure timely and effective translation to clinical settings. To address these challenges, we evaluated the production of microgels using an elliptical pipette for cell encapsulation by droplet emulsion, for high-throughput drug screening. This pipette droplet technology offers significant improvements over traditional droplet emulsion microfluidics, which typically yield micro-organoids at a low rate, require extended culture times, and restrict matrix composition choices. In addition, this method supports automation via liquid handling instruments and is adaptable to both natural and synthetic matrices.
Norbornene-modified hyaluronic acid (NorHA) polymers were synthesized to mimic the extracellular matrix (ECM) and create the cell-encapsulation platform. Spheroids and microgels were fabricated using the NorHA solution, which included crosslinkers DTT, MMP-sensitive motifs, and RGD for cell adhesion, prepared with Irgacure “I2959” as a photoinitiator. Cell encapsulation was performed via water-in-oil emulsion using a custom-deformed elliptical pipette tip. The droplets were polymerized under UV light to form microgels, which were then cultured in optimized conditions for cell proliferation and drug screening. Cell cultures of ovarian (RFP-OVCAR5) and prostate (LNCaP) cancer lines were maintained under standard conditions and used for experiments at a concentration of 4x106 cells/mL. Proliferation and viability assays were performed using mitochondrial activity quantification (WST-1) and confocal microscopy to assess cell growth. For anti-cancer drug screening, encapsulated spheroids were exposed to varying concentrations of doxorubicin and enzalutamide, with IC50 values determined by evaluating cell viability 24 hours post-treatment.
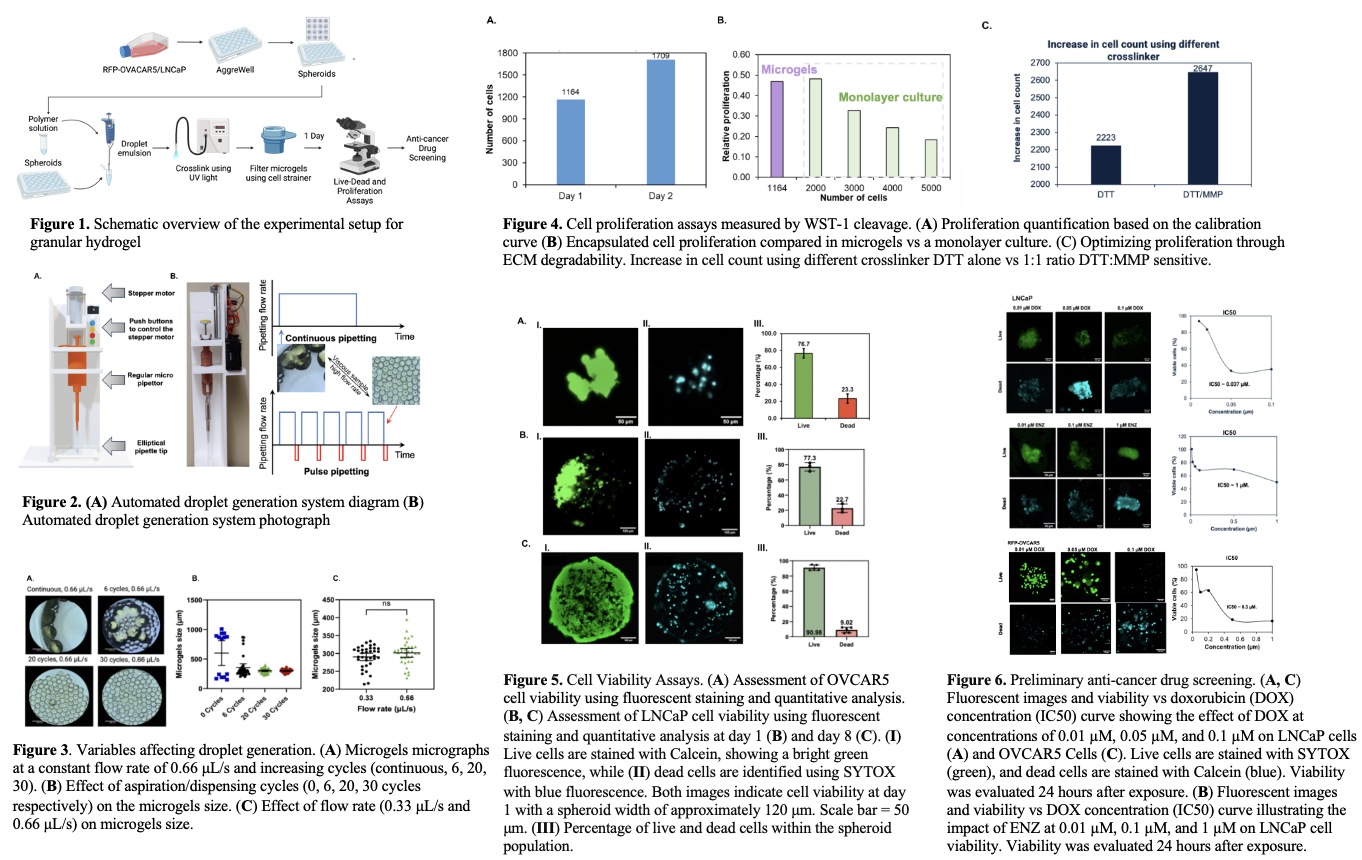
An automated system for pipetting was used to generate microgels and test the effect of pipetting cycles and flow rate on the droplets sizes (Fig. 2). In addition, increasing the flow rate did not have an effect on the uniformity of the droplets, while increasing the aspiration/dispensing cycles greatly improved the uniformity of the droplets (Fig. 3). The mitochondrial activity assessment exhibited an increase in cell count was ~47% (Fig. 4A), with the relative proliferation of encapsulated cells being slightly lower than in monolayer culture (Fig. 4B). A combination of MMP-sensitive and DTT crosslinkers were used in the platform. MMP-sensitive crosslinker is nondegradable and therefore leads to less constraint on the cells. This allows for higher rates of cell proliferation because the cells can modify their ECM providing a more natural environment that can promote tissue growth (Fig. 4C). Viability assays were performed to assess the health and survival of cells within the gel platform before performing drug screening. The increased viability is attributed to proliferation within the platform. The OVCAR5 and LNCaP had 76.7% and 77.3% viability respectively after 24 hours encapsulation (Fig. 5). After 8 days, the LNCaP cell viability increased to 90.98%. The observed increase in viability is attributed to the cell proliferation within the microgels platform. Preliminary cancer drug screening was performed on LNCaP cells using DOX and ENZ at varying concentrations and OVCAR5 cells using DOX. A viability assay and IC50 was constructed. The IC50 values for LNCaP treated with DOX was 0.037 μM, while the LNCaP treated with ENZ had an IC50 value of 1 μM and the OVCAR5 treated with DOX had an IC50 value of 0.3 μM (Fig. 6).
Overall, we successfully developed a versatile microgels platform for high-throughput drug screening that can be applied to various cancer cell lines and tumor microenvironments. This technology demonstrated increased cell proliferation and viability and proved its efficacy in screening under different types of tumor models. Significantly, the findings highlighted the impact of tumor size on drug responsiveness. A plateau in drug effectiveness was observed at higher concentrations, particularly in spheroids larger than 200 µm, suggesting limitations in drug penetration that may result in suboptimal therapeutic outcomes for larger tumors. This highlights the need for precision in drug dosing and selection to enhance treatment efficacy. The scalability of this technology, coupled with potential integration with multi-channel systems positions it as a promising tool for generating patient-derived micro-organoids and conducting personalized drug screening.