2024 AIChE Annual Meeting
Localization of Microparticles in Solution By Microfluidic Interactions on a Patterned Substrate
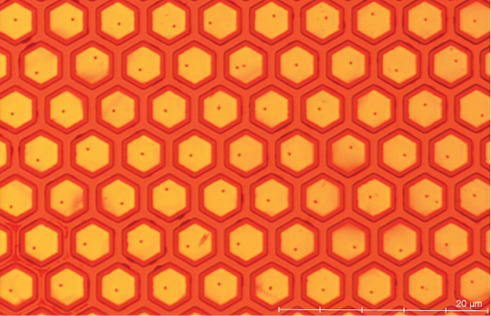
This work develops a fluidic assembly technique to localize microparticles onto a patterned wafer. By patterning a silicon wafer with regions of gold and silicon oxide then thiolating the gold to increase hydrophobicity, microfluidic interactions can localize colloidal particles. A microfluidic channel is formed to press a droplet of nanoparticle solution into a thin film, then the cover is removed causing the bulk fluid to recede and leave behind microdroplets that localize the particles as they evaporate. Localization can also be controlled by altering the solvent properties. For example, adding isopropanol to the primarily aqueous solvent increases surface energy but also decreases the cohesion in the bulk solvent to improve droplet formation. On a thiol-treated gold substrate adding 30% isopropanol by volume gave the highest localization density as shown in Figure 1. This study presents preliminary results and proof of concept for fluidic assembly of microparticles with potential applications in microLED technologies and other microelectronics.
(1) Scholz, S.; Kondakov, D.; Lüssem, B.; Leo, K. Degradation Mechanisms and Reactions in Organic Light-Emitting Devices. Chem. Rev. 2015, 115 (16), 8449–8503. https://doi.org/10.1021/cr400704v.
(2) Chen, L.; Panday, A.; Park, J.; Kim, M.; Oh, D. K.; Ok, J. G.; Guo, L. J. Size-Selective Sub-Micrometer-Particle Confinement Utilizing Ionic Entropy-Directed Trapping in Inscribed Nanovoid Patterns. ACS Nano 2021, 15 (9), 14185–14192. https://doi.org/10.1021/acsnano.1c00014.
(3) Chang, W.; Kim, J.; Kim, M.; Lee, M. W.; Lim, C. H.; Kim, G.; Hwang, S.; Chang, J.; Min, Y. H.; Jeon, K.; Kim, S.; Choi, Y.-H.; Lee, J. S. Concurrent Self-Assembly of RGB microLEDs for next-Generation Displays. Nature 2023, 617 (7960), 287–291. https://doi.org/10.1038/s41586-023-05889-w.
Figure 1: Microscope image of particles localized on a hexagonally patterned substrate.