2024 AIChE Annual Meeting
A High-Throughput Microfluidic Gut-on-a-Chip for Disease Modeling
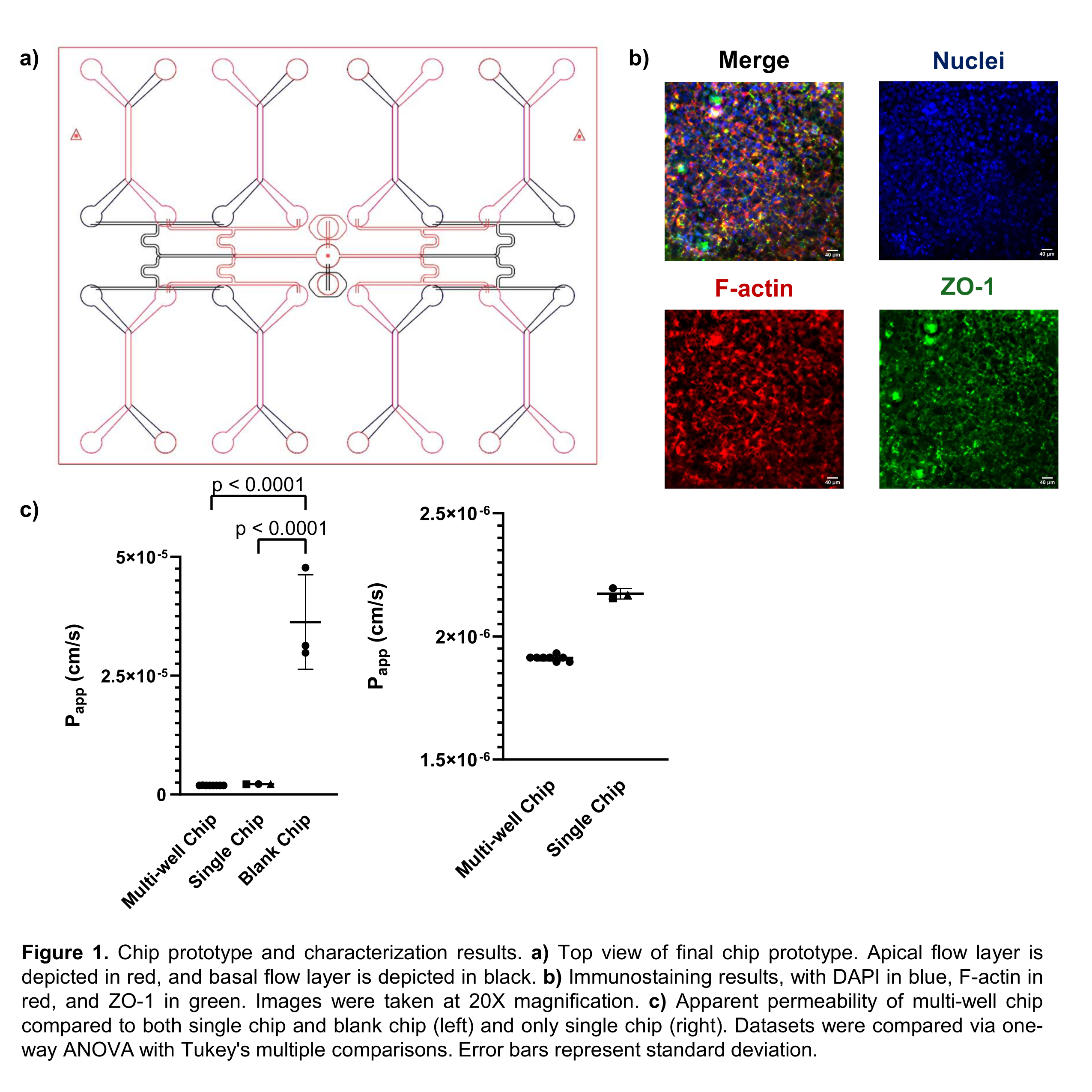
Although OOC have shown promise, current models are low-throughput, requiring extensive time and resources. To address current limitations, we have developed a high-throughput microfluidic gut-on-a-chip (GOC) that is capable of culturing eight independent samples simultaneously. The GOC features a semi-permeable membrane that supports the cell population and forms distinct apical and basolateral compartments for fluid flow, supplying physiologically relevant shear stress. Additionally, our device distributes fluid flow from a single inlet to all eight culture wells, significantly reducing external tubing and pumping requirements (Figure 1a).
Caco-2 epithelial monolayers were grown and matured on-chip over seven days, then the monolayers were fixed and stained for nuclei, cytoskeletal protein F-actin, and tight junction protein ZO-1. Staining images from a representative well indicate high expression of F-actin, which indicates expected morphology of cells, and ZO-1, indicating epithelial maturity (Figure 1b). In addition, a lucifer yellow assay was performed to quantifiably assess the barrier function of each monolayer. The results of this assay indicated a tight distribution of permeability that was significantly lower than a blank chip and not significantly different from a single-chip control (Figure 1c). Thus, the increased throughput does not compromise the overall physiological relevance of the chip.
In summary, our work demonstrates a novel approach to high-throughput culture in OOC, enabling simultaneous culture of eight independent ‘gut samples’ in a single GOC device. Specifically, we applied our device to successfully culture Caco-2 cells on-chip, differentiating to mature epithelial monolayers in all eight culture wells. Ongoing work is focused on applying our high-throughput OOC platform to an in vitro model of IBD, as well as additional organ-chip models.
[1] Lewis, J. D., et al. (2023). "Incidence, Prevalence, and Racial and Ethnic Distribution of Inflammatory Bowel Disease in the United States." Gastroenterology 165(5): 1197-1205 e1192.