2024 AIChE Annual Meeting
Evaluating Gene Integration Efficiency in Ial-PiD2, a Plodia Interpunctella Cell Line
Insect cell lines have demonstrated exceptional potential within applications in vaccine technology, therapeutics, and recombinant protein production. Through genetic modification, insect cells have been engineered to replicate the protein glycosylation patterns of mammalian cells, offering a potentially safer and more cost-effective alternative for cell lines used in the production of human therapeutics requiring these patterns.1 One such example is the Sf9 cell line derived from the pupa ovarian tissue of the fall armyworm, which has been utilized in the baculovirus expression system.2 However, there are over 100 insect cell lines commercially available, yet few have been assessed for applications in the production of recombinant proteins.1 IAL-PiD2 cells derived from the wing imaginal disks of Plodia interpunctella, could provide a valuable platform for recombinant technology. Targeted gene editing using CRISPR/Cas9, and similar technologies can allow for precise and stable genomic integrations, improving the potential for some future applications compared to random genomic integrations seen with piggyBac-based methods. Moreover, the non-viral transfection platform reduces risk to human health should the proteins produced by the cell line have future human applications.1 However, the use of IAL-PID2 cells for protein production has not been evaluated, and research in their genetic manipulation is lacking. Therefore, we aimed to determine the feasibility and efficiency of modifying IAL-PiD2 using tools like CRISPR/Cas9.
Herein, we designed constitutive promoter-driven constructs with the mNeonGreen protein insert for our DNA template, hypothesizing that these constitutive promotor-driven sequences would lead to sustained expression of an easily characterizable phenotype (green fluorescence). IAL-PiD2 cells were seeded in triplicate within a 24 well plate at 100,000 cells per well and reached roughly 65% confluency after 48 hours before transfection with the CRISPR/Cas9 complex with the template. This confluence level was chosen to represent a point where the cells were rapidly dividing while allowing space for continued cell growth during the 7 day period we monitor gene expression intensity.
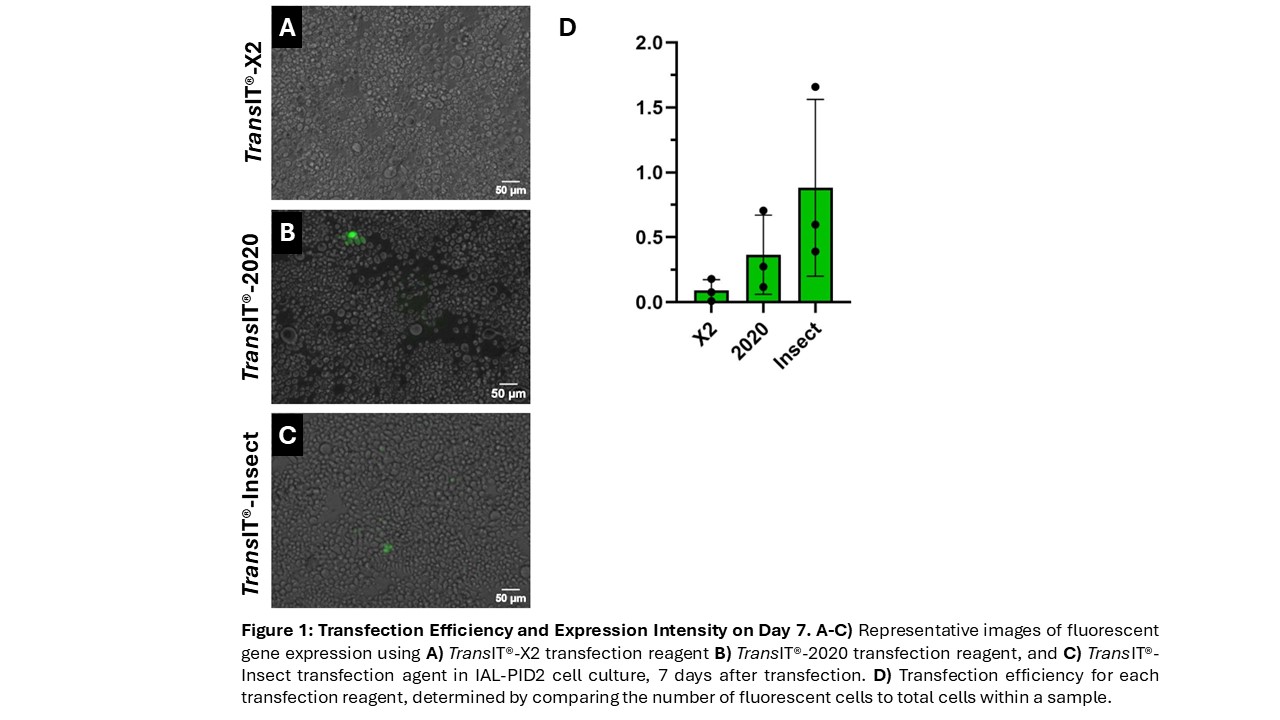
We first sought to evaluate the efficiency of commercially available transfection reagents within the IAL-PiD2 cell line. Three transfection reagents from Mirus Bio® were used to deliver the constructs into the cell line. We analyzed their respective efficiencies in the expression of the mNeonGreen phenotype and evaluated the number of transfected cells and fluorescence intensity over time using fluorescence microscopy. To quantify cell expression and fluorescence intensity across many images, we developed a CellProfiler® pipeline. This software allowed us to count the total cells in each well and quantify the cells expressing the desired phenotype by setting desired threshold limits for fluorescence intensity. Results show increased transfection efficiency over time with substantial expression beginning on Day 5 after transfection. Based on Day 7 data, TransIT®-Insect, the transfection reagent synthesized for insect cell lines, was the most efficient (Figure 1). Future work aims to compare transfection efficiency in SF9 cells with the results obtained in IAL-PiD2 cells.
- Yee CM, Zak AJ, Hill BD, Wen F. The coming age of insect cells for manufacturing and development of protein therapeutics. Industrial & engineering chemistry research. 2018;57(31):10061-70.
- Hong Q, Liu J, Wei Y, Wei X. Application of Baculovirus expression vector system (BEVS) in vaccine development. Vaccines. 2023;11(7):1218.