2024 AIChE Annual Meeting
Effects of Embryonic Hyperglycemia on Early Stage Embryo Development
To further explore the effects of embryonic hyperglycemia on the development of the embryo, avian models were exposed to high levels of glucose before incubation. A 500mM D-glucose solution was injected into the yolk at a volume of 300uL (D300) or 600uL (D600) before the egg was incubated. Control groups were similarly dosed: 600µL of a 500mM L-Glucose solution (L600) served as the osmotic control, and 600µL of saline (saline) was used as a vehicle. Untreated (Control) eggs were pierced with a needle but were not injected with any solution to account for the effects of the injection itself.
Eggs were opened on day 3, then the embryonic heart was imaged and velocity of blood through the primitive heart outflow tract of the heart was measured with optical coherence tomography (OCT). The viability was tracked daily through day 9.
At Day 3, D600 embryos died at a higher rate than control embryos (Table 1), suggesting that glucose additionally impacts their development early on. By day 7 of incubation, physical defects were observed in D600 embryos compared to control embryos. Defects include not developing organs or being under-developed.
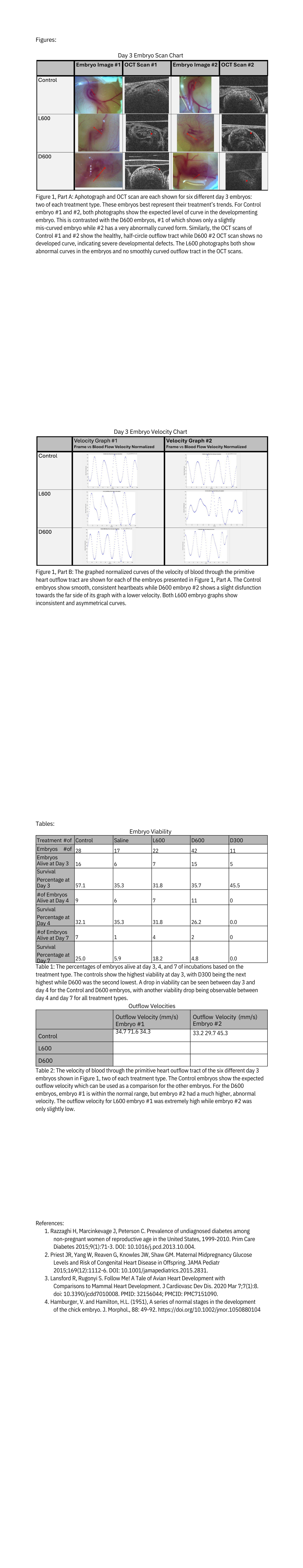
Viable embryos were imaged and staged at day 3 and their development observed (Figure 1, Part A). Using Hamburger-Hamilton (HH) Stages, a common method for staging chicken development, as a guideline, a trend was found with D600s embryos generally showing underdevelopment (Figure 1, Part A) 4. Both control embryos are at expected development stages. D600 embryo #1 was slightly underdeveloped and D600 embryo #2 was too deformed to determine how developed it was (Figure 1, Part A). Both L600 embryos showed to be maldeveloped (Figure 1, Part A) but only those two day 7 L600 embryos were photographed so it is difficult to determine a trend in L600 from this.
From the OCT scans, the L600 scans both show additional noise and malformed curves (Figure 1, Part A). D600 embryo #1 did show a clear outflow tract (Figure 1, Part A) and D600 embryo #2 showed extreme malformations and no clear outflow tract. Both controls show the expected smooth curve of primitive heart outflow tract, which is the expected development (Figure 1, Part A).
Both control embryos showed normal outflow velocity graphs when normalized (Figure 1, Part B) and outflow velocities (Table 2) within the same range as those found by previous studies 3. The L600 embryos both showed abnormal graphs, with the graph trends being inconsistent (Figure 1, Part B), and abnormal velocities (Table 2). For the D600 embryos, both graphs showed to be fairly normal (Figure 1, Part B) and the outflow velocity of embryo #1 was within the normal range, but embryo #2 had a higher, abnormal velocity (Table 2).
The effects of embryonic hyperglycemia in avian models become apparent by day 3 of incubation. Future exploration of this topic could include running trials using D300 instead of D600 to determine if the same defects would still occur. If the results are similar, the D300 would offer a higher viability method to study the defects caused by embryonic hyperglycemia. Regardless of how the topic is explored in the future, prioritizing the understanding of PGDM pregnancy risks could ultimately aid both expectant parents and their offspring.