2024 AIChE Annual Meeting
Cycling Effects & Stability of Polythiophene-Coated Lfp Cathode Current Collectors
As global initiatives to mitigate anthropogenic carbon dioxide contributions
intensify, electrochemical storage systems are poised to play an integral role in
the eco-friendly metamorphosis of electricity and transportation. Lithium- ion
battery (LIB) have a high energy density and low self-discharge compared to
other batteries utilized within electric vehicles, making them the most common
battery. While advances in LIB technologies continue, significant obstacles to
LIB adoption persist in the high-demand grid and transportation sectors, such as
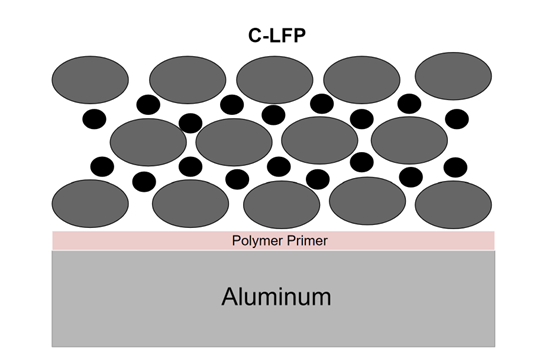
within extending their useful life. This study explores the use of polythiophene
and its derivatives (poly(3-butylthiophene) (P3BT), poly(3-hexylthiophene)
(P3HT), and poly(3-octylthiophene (P3OT)) as priming materials coated on the
aluminum cathode current collector surface in Lithium Iron Phosphate (LFP) LIB.
The use of an electroconductive polymer would ensure good contact between
the active materials and conductive additive and the current collector, which
would protect the electrode from corrosion through its cycling. This method is
expected to enhance battery stability by preventing electrode materials
decomposition, thereby extending battery life. Additionally, the high electronic
conductivity of the polymers is predicted to improve cycling ability, resulting in
increased Coulombic efficiency, and rate capacity. There is also evidence for the
improved stabilization of the Cathode Electrolyte Interphase (CEI) during the first
several cycles that would create a protect layer outside the active material, and
further limit side reactions. This preparation technique holds promise for
enhancing EV battery performance and longevity, with the prospect of future
research for the optimization of the technique.
intensify, electrochemical storage systems are poised to play an integral role in
the eco-friendly metamorphosis of electricity and transportation. Lithium- ion
battery (LIB) have a high energy density and low self-discharge compared to
other batteries utilized within electric vehicles, making them the most common
battery. While advances in LIB technologies continue, significant obstacles to
LIB adoption persist in the high-demand grid and transportation sectors, such as
within extending their useful life. This study explores the use of polythiophene
and its derivatives (poly(3-butylthiophene) (P3BT), poly(3-hexylthiophene)
(P3HT), and poly(3-octylthiophene (P3OT)) as priming materials coated on the
aluminum cathode current collector surface in Lithium Iron Phosphate (LFP) LIB.
The use of an electroconductive polymer would ensure good contact between
the active materials and conductive additive and the current collector, which
would protect the electrode from corrosion through its cycling. This method is
expected to enhance battery stability by preventing electrode materials
decomposition, thereby extending battery life. Additionally, the high electronic
conductivity of the polymers is predicted to improve cycling ability, resulting in
increased Coulombic efficiency, and rate capacity. There is also evidence for the
improved stabilization of the Cathode Electrolyte Interphase (CEI) during the first
several cycles that would create a protect layer outside the active material, and
further limit side reactions. This preparation technique holds promise for
enhancing EV battery performance and longevity, with the prospect of future
research for the optimization of the technique.

