Despite significant progress in HIV treatment since the virus was discovered 40 years ago, around 38 million people worldwide still live with HIV, and the virus continues to cause nearly 700,000 deaths annually. A significant barrier to a cure is the presence of latent viral reservoirs in memory T cells, necessitating lifelong therapy. Current HIV treatments predominantly rely on nucleoside reverse transcriptase inhibitors like lamivudine (3TC), but these drugs have low molecular weight and are hydrophilic, leading to rapid systemic clearance and frequent dosing. When doses are missed or adherence decreases, the virus can mutate and progress to AIDS, where the immune system is severely compromised and life expectancy is significantly reduced.
The primary challenge in developing long-acting injectables for antiviral treatment is extending the drug release span. To address this, we exploit the reversible properties of peptide-based supramolecular polymers and their unique ability to self-assemble into filamentous nanostructures in aqueous environments via non-covalent interactions and form three-dimensional hydrogel networks under physiological conditions. Developing self-assembling drug amphiphiles (DAs), where therapeutic agents are chemically linked to peptide amphiphiles and incorporated into hydrogels, represents a novel approach to controlling drug release through controlling the kinetics of dissociation from the supramolecular state to the monomeric state and achieving sustained therapeutic effects.
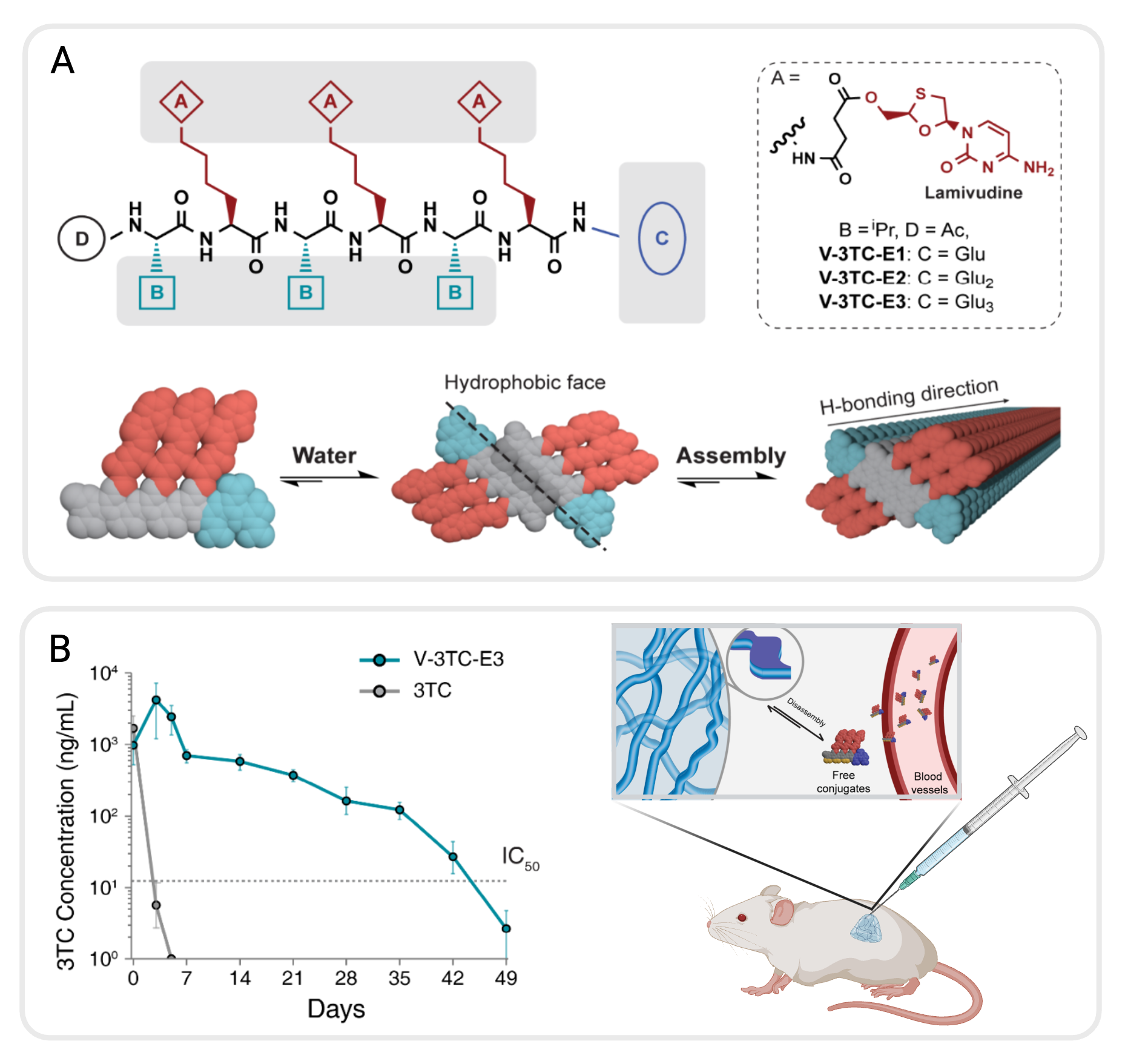
We designed a series of ARV DAs based on lamivudine, a hydrophilic antiretroviral (ARV) drug and nucleoside reverse transcriptase inhibitor. These ARV DAs are constructed with alternating sequences of hydrophobic valine (V) and hydrophilic lysine residues (K) connected through a biodegradable succinic linker to lamivudine (K3TC), with varying numbers of negatively-charged glutamic acid (E) residues (1–3) at the C-terminus. When dissolved in deionized water, the ARV DAs spontaneously self-assembled into micrometer-length supramolecular filaments with varying degrees of lateral stacking: the increasing number of E residues provides enough electrostatic repulsion to maintain assembly integrity and enhances water-soluble ARV DAs. Adding 1× PBS caused rapid gelation in the ARV DAs that contained 2 or 3 E residues—in vitro studies revealed that dilution caused the dissociation of the filaments into monomers, enabling linear, sustained, long-term drug release. In vivo experiments further validated the injectability of these ARV DAs, their rapid in situ hydrogel formation upon injection, enhanced local retention at the injection site, and prolonged therapeutic release over a month. Pharmacokinetic data indicated that these supramolecular hydrogels maintained plasma levels of lamivudine above the IC50 for over 40 days in mice, with minimal inflammation and adverse immune response. These results provide important insights into the strategic design of long-acting injectable therapies through drug-based molecular assembly approaches, and the ARV supramolecular hydrogels demonstrated here show great potential for enhancing HIV treatment outcomes.