2024 AIChE Annual Meeting
(86e) CO2 Hydrogenation to Methanol Using Hybrid Flame-Made CuO/ZrO2 – Polymer Membrane Reactors
Authors
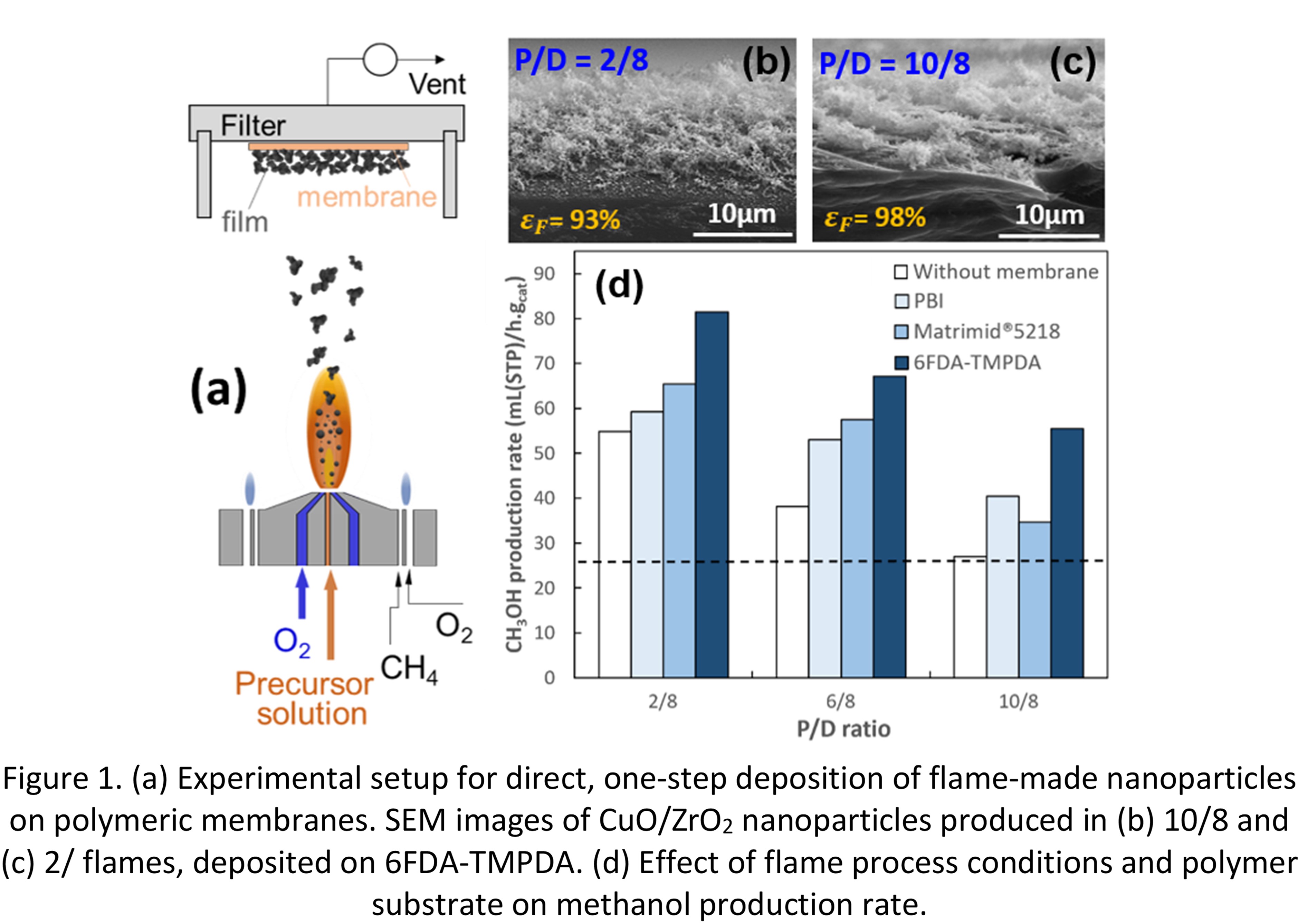
In response to this challenge, hybrid nanoparticle-membrane nanocomposites are manufactured in this work via direct one-step deposition. CuO/ZrO2 nanoparticles are produced by flame spray pyrolysis (FSP) at various precursor solution-to-oxygen (P/O) flow rate ratios and are deposited on polymeric membranes highly selective to methanol (Figure 1a). Figure 1b and c shows scanning electron microscopy (SEM) images of flame-made CuO/ZrO2 catalyst produced at a P/D ratio of (b) 2 mL/min precursor solution to 8 L/min oxygen (2/8, cold flame) and (c) 10/8 (hot flame), deposited on 6FDA-TMPDA membranes. Increasing the precursor flow rate, leads to higher temperature and longer high-temperature particle residence time, which in turn results in larger CuO and ZrO2 crystal size and smaller specific surface area.
The effect of nanoparticle and film characteristics on the CO2 conversion and methanol production rate is quantified. FSP conditions leading to nanocatalysts with highly activity to CO2 hydrogenation are identified, while the selectivity to methanol, which is enhanced by the presence of polyimide membranes, is also evaluated. At 200 °C and 20 bar, these catalytic membrane reactors demonstrate exceptional performance, achieving 113% improvement in carbon dioxide conversion and a remarkable 106% increase in methanol production rate (Figure 1d) compared to conventional catalytic fixed bed reactors (Fig. 1d: horizontal line). Overall, the catalyst-membrane reactors have shown long-term stability and ability to suppress catalyst deactivation caused by water, ensuring sustained catalyst stability throughout the reaction.
Such hybrid membrane-catalytic nanoparticle reactors are a promising technology which enables industries to convert their carbon dioxide wastes into valuable energy products, such as methanol.
Funded by Future Fuels Cooperative Research Centre grant (RP1.3-04) and the University of Melbourne, Australia.
References
Kattel, S., Yan, B., Yang, Y., Chen, J. G., and Liu, P. (2016). J. Am. Chem. Soc. 138: 12440-12450.
Witoon, T., Numpilai, T., Phongamwong, T., Donphai, W., Boonyuen, C., Warakulwit, C., Chareonpanich, M., and Limtrakul, J. (2018). J. Chem. Eng. 334: 1781-1791.
Tada, S., Watanabe, F., Kiyota, K., Shimoda, N., Hayashi, R., Takahashi, M., Nariyuki, A., Igarashi A., Satokawa, S. (2017). J. Catal. 351: 107-118.