Chemical recycling, particularly through pyrolysis, has emerged as a promising solution for plastic waste management. It has the potential to process both single and mixed plastics, transforming them into value-added chemicals, monomers, and fuels. Polymer pyrolysis results in complex reaction networks involving thousands of species and reactions. Factors such as temperature, residence time, heating rate, and sample characteristics influence product distribution and play vital roles in kinetic measurements as well as the extent of secondary reactions. Polymer samples, particularly those with large length scales, such as packed powders and pellets, can significantly impact kinetics due to heat and mass transfer rates within the polymer sample. This underscores the importance of selecting the appropriate reactor configuration and establishing well-defined experimental conditions to minimize the transport limitations.
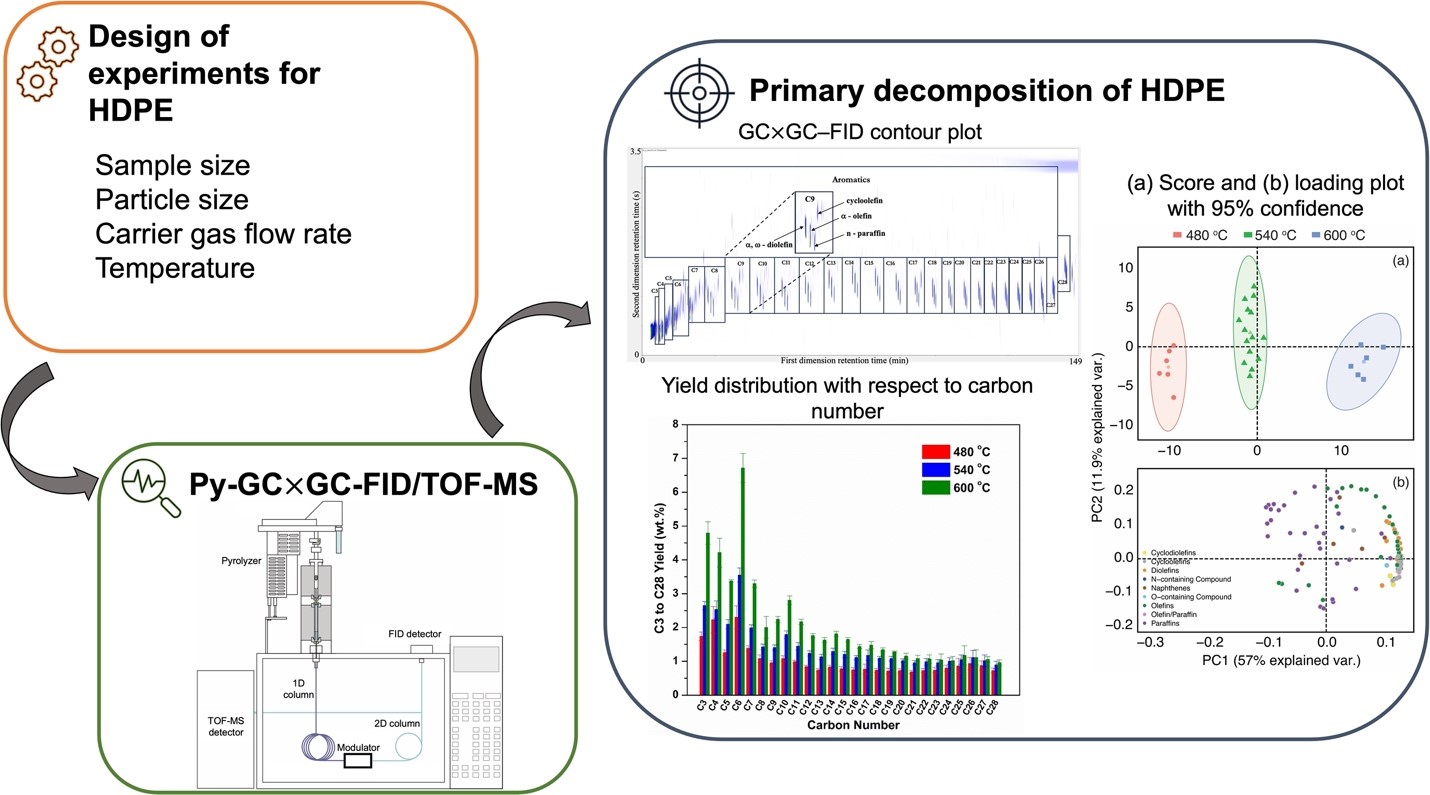
Using a combination of well-defined and well-controlled experimentation, data science, and advanced analytics, this work methodically studied the effect of various pyrolysis variables on the product distribution of high-density polyethylene (HDPE) to validate the pyrolysis conditions that minimize transport effects and secondary gas-phase reactions. The primary decomposition of HDPE was performed using a Box−Behnken design to evaluate the role of pyrolysis variables such as particle size, sample size, carrier gas flow rate, and temperature. The pyrolysis of HDPE under various experimental conditions resulted in the identification of nearly 500 products using GC X GC/TOF−MS and quantification with FID. The chemical groups identified for the HDPE pyrolysis included paraffins, olefins, diolefins, and cyclic and aromatic hydrocarbons among others. The film
thickness calculated using characteristic time analysis for the sample sizes (50−150 μg) employed in this work varied in the range of 4.7−14.1 μm. The analysis indicated that the experiments conducted within the BBD space were well within the isothermal, reaction-controlled regime. Principal component analysis (PCA) demonstrated statistical differences for temperature (480−600 C), while no statistical differences were observed for particle size (53−125 to >300 μm), sample size (50−150 μg), and carrier gas flow
rate (100−300 mL min−1). The total yield of C3 to C28 increased from 25.98 1.28 to 55 1.42 wt % when temperature was increased from 480 to 600 C. When the sample size of 1000 μg was considered for pyrolysis, PCA analysis showed statistical differences in the product distribution with respect to the smaller sample sizes of 50− 150 μg, which can possibly indicate the presence of transport effects at such higher sample sizes. This work underscores the importance of carefully selecting the pyrolysis parameters to curtail secondary gas-phase reactions and minimize transport effects. The product speciation data obtained under such experimental conditions are valuable for developing microkinetic models for polymer pyrolysis.