2024 AIChE Annual Meeting
(735w) Molecular Dynamics Study of Methane Hydrate Dissociation Under Silica Confinement: Role of Salinity
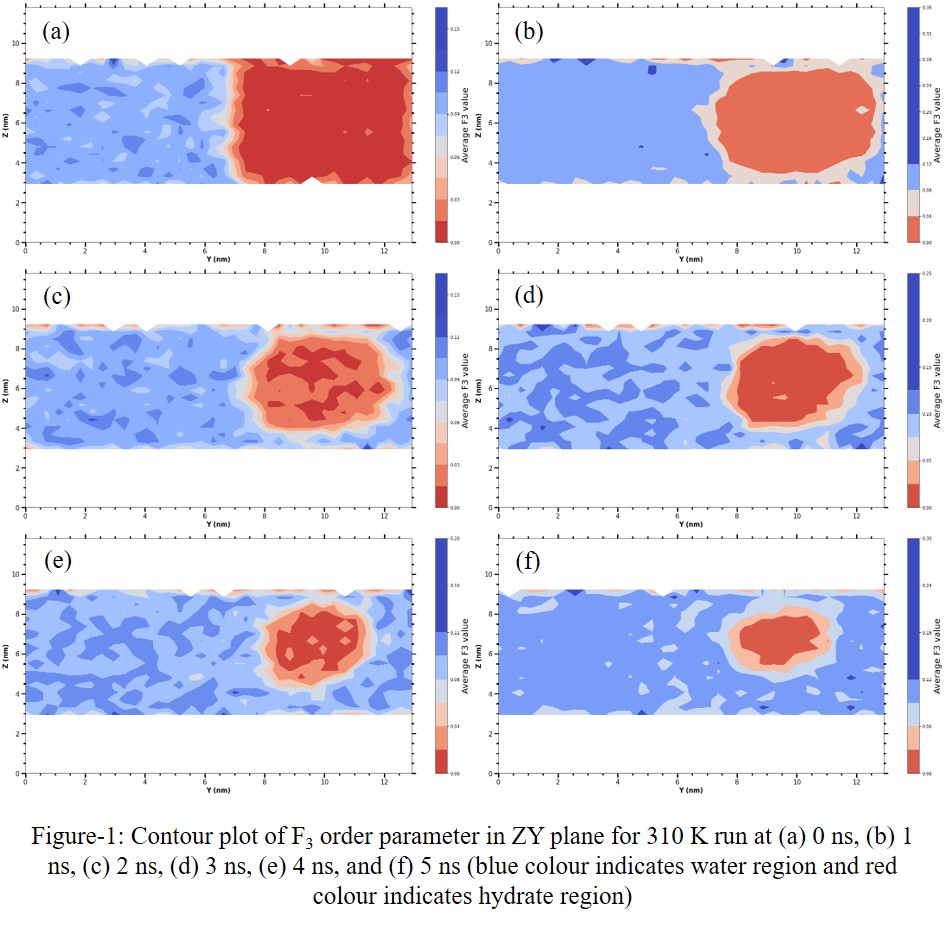
In nature, CH4 hydrate reservoirs are found in the seafloor and permafrost regions. In most of the reservoirs, gas hydrates are present in pores of sedimentary rocks which are of silica and carbonate types. Bagherzadeh et al. in their work found that the bulk phase dissociation of CH4 hydrate is faster compared to dissociation under silica confinement. They also showed that the presence of a bound layer near the silica surface limits the interactions between the surface hydroxyl group and hydrate water molecules so an intermediate dissociation rate is obtained in that case. [1] Simulations performed by Fang et al. showed that the silica layer provides adsorption sites to CH4 nanobubble which limits the migration of nanobubble from pore to bulk phase. [2] This indicates the deviation of hydrate properties under confinement compared to the bulk phase and hence confinement will also affect the recovery process. Thus, it is of vital importance to strengthen our understanding of gas hydrate behaviour under confinement to represent actual reservoir scenarios and improve the efficiency of methane extraction from hydrate reservoirs. Towards this goal, we have performed molecular dynamics (MD) simulations of the CH4 hydrate phase in contact with liquid water which is placed under the confinement of silica walls. We have considered three systems: (1) liquid water – hydrate (Lw-H) bulk phases (2) Lw-H phases under silica confinement (3) Lw-H phases under confinement with NaCl added in aqueous phase. The number of NaCl atoms in the system corresponds to the ~3.5 wt % concentration of NaCl in water which is near to the seawater conditions. [3] Unlike the previous confinement simulations reported in the literature [1,2,3] silica atoms in our simulations are neither position restrained nor kept frozen during the MD run; we have allowed silica atoms to move freely according to their natural dynamics based on interactions calculated from selected forcefields. Simulations have been performed in the constant volume and temperature (NVT) ensemble at 290, 300, 305, and 310 K temperatures. The choice of these temperatures is based on our findings from the bulk Lw-H phase melting point of CH4 gas hydrate for a combination of TIP4P/2005 model of water and TraPPE-EH model. Through these simulations, we want to study mainly four aspects which are: (1) confinement effect on methane hydrate dissociation, (2) effect of temperature on hydrate dissociation under confinement, (3) effect of salinity, i.e., the addition of NaCl to the liquid phase on methane hydrate dissociation, and (4) nanobubble interaction with the silica surface. On comparing these results to Lw-H phases under confinement, we find that when Lw-H phases are confined in silica slab, melting point suppression occurs. From our bulk phase simulations, we observe that the methane hydrate dissociates at 310 K temperature while the hydrate phase remains stable at other temperatures i.e., 290, 300, and 305 K. Apart from that, rate of hydrate dissociation at 310 K is much faster under confinement; such faster dissociation under confinement can be explained by the interaction of surface -OH groups of silica slab with hydrate water molecules via the hydration shell which affects the hydrogen bonding of hydrate structure near the silica slab. From the contour plots of the F3 order parameter, we observe hydrate dissociation occurring in a shrinking core manner [1] (refer to Figure-1). We observe the formation of methane nanobubble (either cylindrical or spherical in shape) in our simulations. Interestingly we notice that regardless of the shape of the nanobubble, the nanobubble formed in all cases tends to go towards the slab substrate and stick to the surface. A similar observation is also reported by some of the previous confinement simulation work. [2] Also, we notice the formation of an ordered bound water layer on the hydrophilic silica slab under the methane nanobubble. Water molecules in this bound water layer are ordered in square lattice form whereas in other parts of the silica surface there is no specific ordering of water molecules. This raises an interesting question: “Does the tendency of the nanobubble to adhere to the silica surface affect the recovery of methane?” Answering these questions will help to enhance our understanding of the overall methane recovery process at reservoir conditions.
References:
[1] Bagherzadeh, S.A., Englezos, P., Alavi, S. and Ripmeester, J.A., 2012. Molecular modeling of the dissociation of methane hydrate in contact with a silica surface. The Journal of Physical Chemistry B, 116(10), pp.3188-3197.
[2] Fang, B., Ning, F., Ou, W., Wang, D., Zhang, Z., Yu, Y., Lu, H., Wu, J. and Vlugt, T.J., 2019. The dynamic behavior of gas hydrate dissociation by heating in tight sandy reservoirs: A molecular dynamics simulation study. Fuel, 258, p.116106.
[3] B. Fang, T. Lü, L. Cheng, D. Wang, Y. Ni, B. Fan, J. Meng, T.J.H. Vlugt, and F. Ning, “Negative Effects of Inorganic Salt Invasion on the Dissociation Kinetics of Silica-Confined Gas Hydrate via Thermal Stimulation,” Energy Fuels 36(12), 6216–6228 (2022)