Breadcrumb
- Home
- Publications
- Proceedings
- 2024 AIChE Annual Meeting
- Separations Division
- Crystallization Process Development
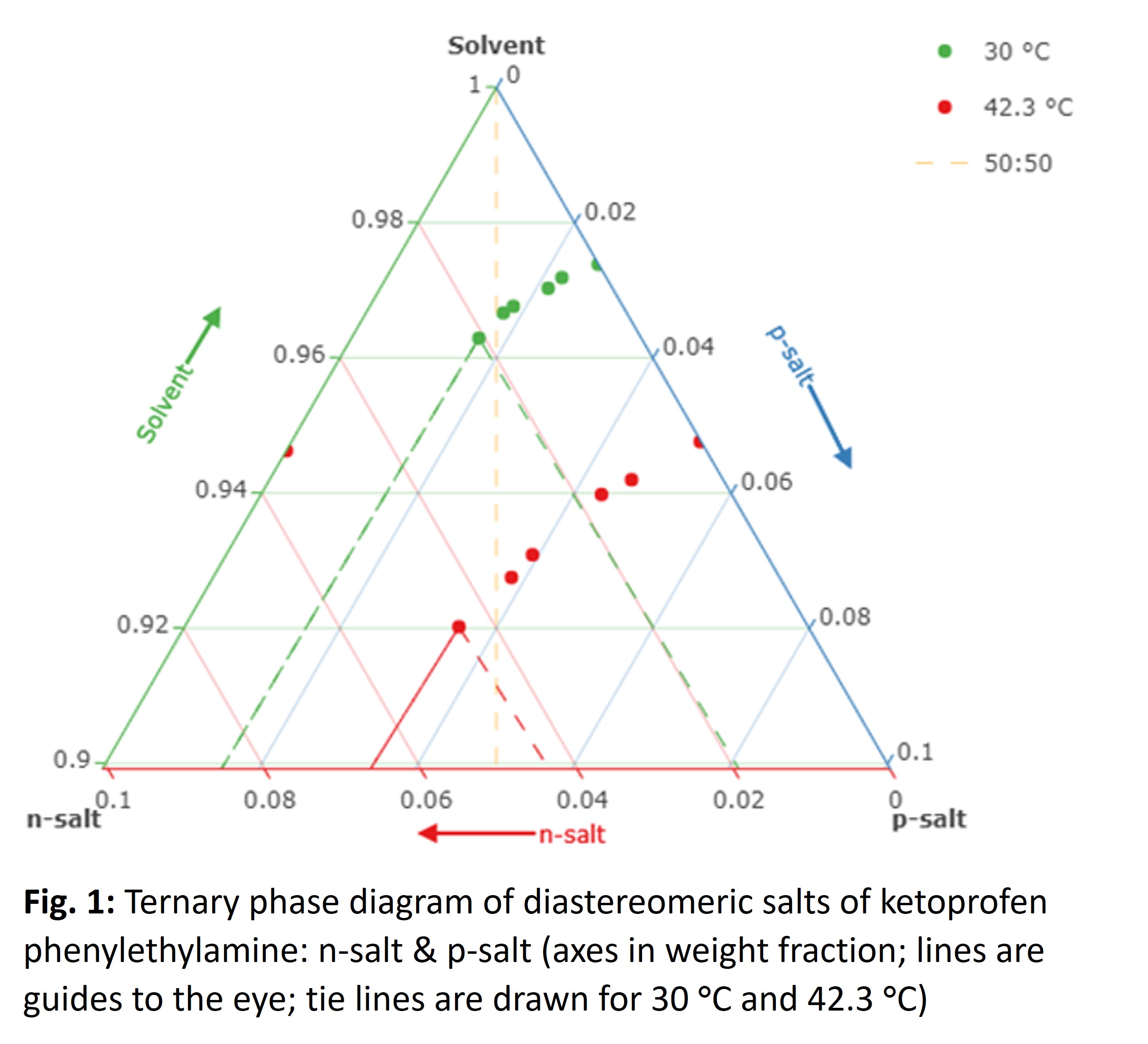
- (727b) Diastereomeric Salt Crystallization Using Ternary Phase Diagram: A Ketoprofen Phenylethylamine Case Study

One approach for isolating a high-purity enantiomer from a racemic mixture involves the formation of diastereomeric salts. This method entails reacting racemic acidic or basic target compounds with optically active compounds, such as enantiopure basic or acidic resolving agents, to produce a pair of diastereomeric salts. These salts can then be separated through fractional crystallization. Following this separation, the resulting salt undergoes a neutralization process to yield a pure enantiomer (Simon, M. et al, 2018).
The diastereomeric salt formation of S- ketoprofen-S- (1)-phenylethylamine (p-salt) and R- ketoprofen-S- (1)-phenylethylamine (n-salt) was systematically investigated by examining binary and ternary phase diagrams, utilizing a solvent consisting of 50:50 wt.% IPA: Heptane. The eutectic point for the salt composition occurs at approximately 56:44 wt.% n-salt: p salt. Subsequently, based on this finding, a crystallization procedure was developed to achieve enantiopure separation of the pure p-salt, utilizing information from the ternary phase diagram (Fig. 1).
Acknowledgements: The authors acknowledge the support of Enterprise Ireland under the Research, Development and Innovation Fund, Grant award 180753/RR.
Simon, M., Donnellan, P., Glennon, B. and Jones, R.C. 2018. Resolution via diastereomeric salt crystallization of ibuprofen lysine: ternary phase diagram studies. Chemical Engineering & Technology, 41(5), pp. 921-927.