2024 AIChE Annual Meeting
(727a) Enantioselective Crystal Flotation of Racemic Compound Forming Systems
Authors
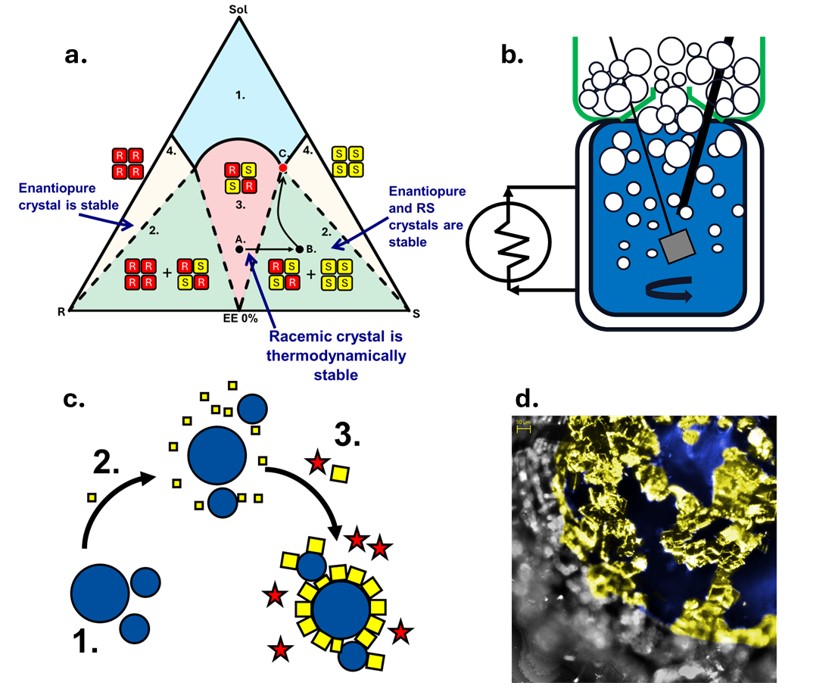
Preferential crystallization, where one enantiomer is directly crystallized via seeding while the counter enantiomer crystal is kinetically inhibited, has gained a lot of interest as a cheap, inexpensive technique. Unfortunately, research in this area predominantly focuses on only 5-10% of chiral systems which crystallize as conglomerates, where the enantiomers form individual enantiomerically pure crystals. However, ~90% crystallize as racemic compound forming systems where the enantiomers crystallize together in a 1:1 ratio within the unit cell.8 The presence of the racemic crystal complicates the thermodynamics of the system resulting in a two-step process. A solution with an enantiomeric excess (EE) 0% must be enriched in one enantiomer to move the system into the two-phase region of the ternary phase diagram (TPD).9 In this region, both the racemic crystal and the enantiopure crystal can form. The racemic crystal nucleation and growth can be kinetically inhibited and an enantiopure product can be produced. As current research is limited to conglomerate systems, often methods based on this separation are applied to racemic crystals which overlooks unique properties that majority of chiral systems may have.
In this work, we present the discovery of a unique phenomenon that allows for the selective interaction of bubbles with enantiomeric crystals and racemic crystals due to the minor differences in surface energy of these crystals. We demonstrate that enantiopure and racemic crystal of Mandelic acid have different surface properties such as hydrophobicity and surface energy and, therefore, exhibit different sorption interactions with small bubbles. Thus, an enantiomeric crystal flotation (ECF) process was developed that could enhance solution EE from 50% to 92% in the foam product.
The process involved sparging bubbles into a jacketed vessel during crystallization where dissolved, pulp and foam phases were monitored. The dissolved phase was tracked via an online densitometer and polarimeter. While pulp and foam phases were monitored offline by gravimetric analysis and HPLC. A complete mass balance of the system was performed which tracks the selective removal of the enantiopure crystal from the racemic crystal. Crystallization and flotation kinetics for both the racemic and enantiopure crystal were individually examined to determine key process parameters, such as bubble size and quantity as well as crystal size and morphology.
The application to other racemic compound forming systems was explored and a number of techniques were employed to find a suitable methodology for screening. While a number of parameters affect flotation rate and recovery, the most important parameter is the surface energy difference between racemic and enantiopure crystals. Surface energy (SE) and hydrophobicity were quantified via contact angle measurements, inverse gas chromatography (IGC) and nanocontact angle measurements, performed using atomic force microscopy. All results show the enantiopure S-Mandelic acid crystal having the lowest SE and greatest contact angle. IGC was determined as the simplest method to accurately determine the SE difference where a difference of 4mJ/m2 was observed between crystal of RS-Mandelic acid and S-Mandelic acid.
The separation is limited to the region in the ternary phase diagram where both the racemic and enantiopure crystal can form. Therefore, an enrichment step is still necessary to move the initial solution from EE 0%, where the racemic crystal is thermodynamically favorable. However, this enantiospecific spatial separation technique presents an interesting mechanism for selective removal and future application. If it is possible to instead kinetically drive the formation of the enantiopure crystal in an undesirable region of the TPD, the ECF process would allow for its immediate removal before its redissolution and conversion to the thermodynamically stable RS crystal.
References
1 L. A. Nguyen, H. He and C. Pham-Huy, Int. J. Biomed. Sci., 2018, 49, 1116–1155.
2 N. M. Maier, P. Franco and W. Lindner, J. Chromatogr. A, 2001, 906, 3–33.
3 J. M. Hawkins and T. J. N. Watson, Angew. Chemie - Int. Ed., 2004, 43, 3224–3228.
4 P. R. Anandamanoharan, P. W. Cains and A. G. Jones, Tetrahedron Asymmetry, 2006, 17, 1867–1874.
5 C. Fernandes, M. E. Tiritan and M. M. M. Pinto, Symmetry, 2017, 9
6 E. Francotte, T. Leutert, L. La Vecchia, F. Ossola, P. Richert and A. Schmidt, Chirality, 2002, 14, 313–317.
7 A. Rajendran, G. Paredes and M. Mazzotti, J. Chromatogr. A, 2009, 1216, 709–738.
8 F. Cascella, A. Seidel-Morgenstern and H. Lorenz, Chem. Eng. Technol., 2020, 43, 329–336.
9 H. Lorenz, E. Temmel and A. Seidel-morgenstern, in Handbook of Continuous Crystallisation, The Royal Society of Chemistry, 2020, pp. 422–468.
Figure 1a. Simplified of Ternary Phase Diagram (TPD) for Racemic Compound Forming system b) Enantioselective Crystal Flotation Process c) Crystallization and selective bubble adsorption d) Microscopic Image of enantiopure Mandelic acid crystal adsorption on bubble surface