2024 AIChE Annual Meeting
(708f) Electron Paramagnetic Resonance (EPR) Spectroscopy As a Versatile Tool in Determining Radical Kinetics in Photocatalytic Reactions.
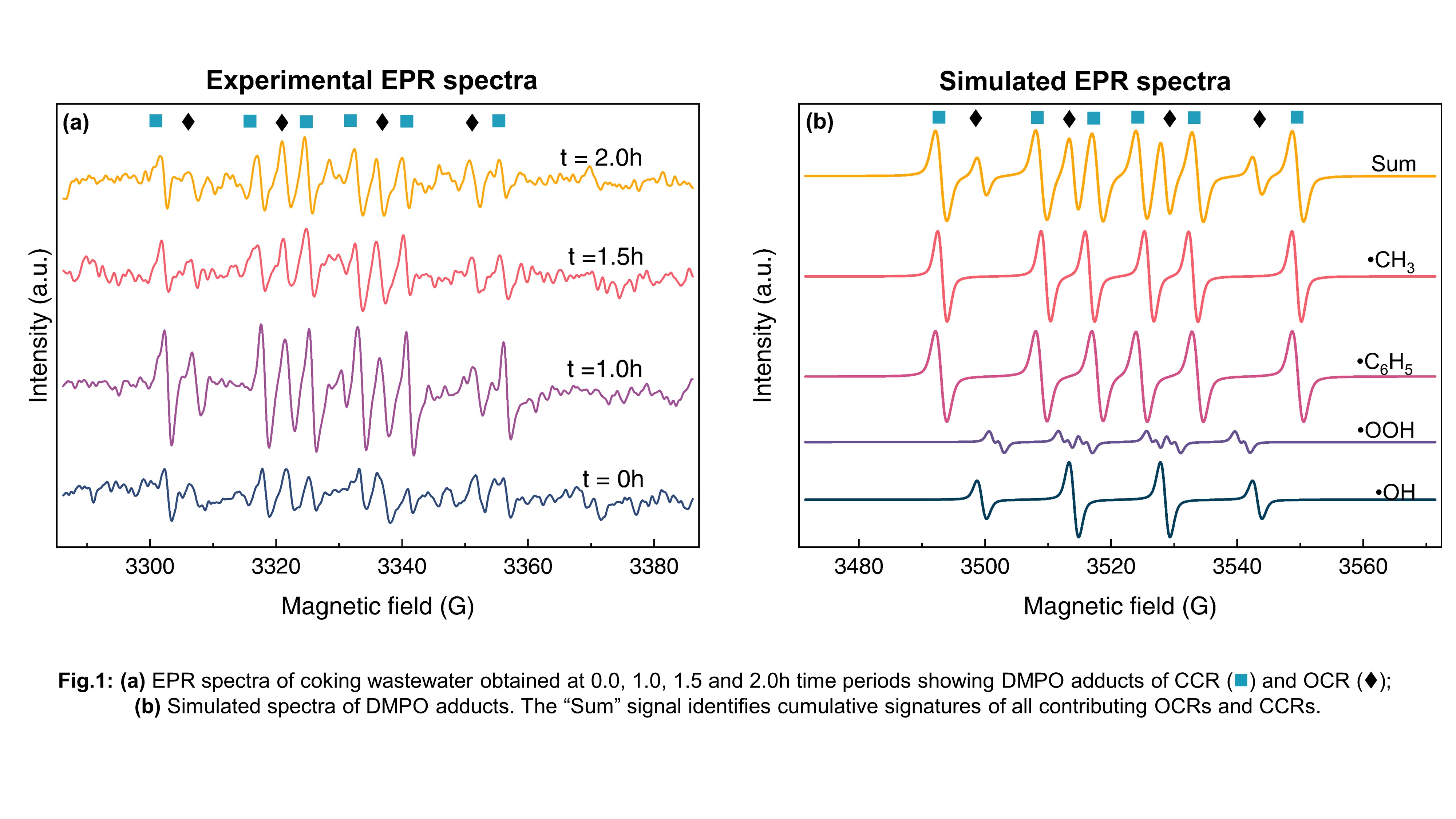
Most photocatalytic reactions follow radical exchange mechanism as the framework for redox reactions. Oxygen centered radicals (OCR) generated in-situ through photo-mediated water splitting and carbon centered radicals (CCR) formed as degradation by-products exhibit paramagnetic states within the reaction cycles. Unlike ex-situ techniques such as mass spectrometry which identify metastable intermediates by breaking them into smaller ion fragments, in-situ techniques such as EPR can directly probe short-lived metastable radical intermediates and quantify them. In our work, we apply EPR to identify the dominant OCRs such as hydroxyl radicals generated by our in-house developed S-scheme Ag/AgBr/TiO2 visible-light photocatalyst. The as-prepared photocatalyst was used to oxidize polycyclic and heteroaromatic compounds in industrial coking wastewater. We further quantified the radicals by simulating their EPR signals on EasySpin and curve fitting the time variant EPR spectra of OCRs and CCRs occurring in the aqueous media. 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) was used as the trapping agent to identify spin adducts of hydroxyl (DMPO-OH), hydroperoxyl (DMPO-OOH), phenyl (DMPO-C6H5) and alkyl (DMPO-R) radicals. Maximum free radical concentration was observed at 1h followed by a marked decrease. This could be attributed to the radicals participating in the redox mechanism dominating the degradation of aromatics. Results of theoretical kinetic modelling showed excellent agreement with radical concentrations obtained through integrated EPR signals. Through quenching studies, we determined valence band generated holes (hVB+) as the dominant photoredox species. Varying radical concentrations led to the generation of different aromatic and aliphatic byproducts in coking wastewater which was confirmed by headspace gas chromatography. For the first time, EPR has been used to track the temporal rise and decay of radicals simultaneously participating and being produced in photocatalytic degradation. This work lays the fundamental background in detecting reaction kinetics of complex reactant media indirectly through EPR.