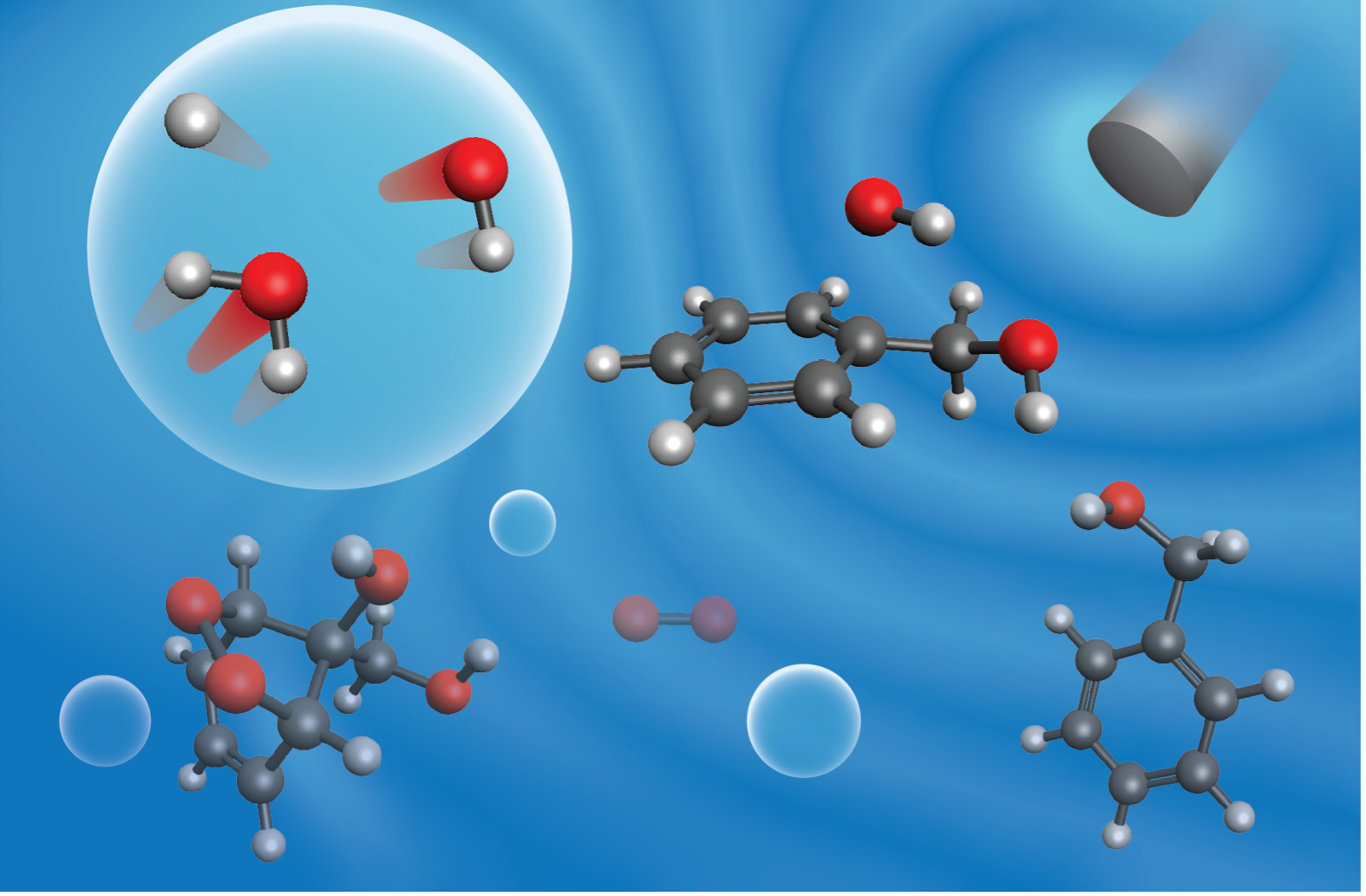
Ultrasound irradiation forms •OH in aqueous solutions by driving H
2O homolysis in cavitating bubbles. These powerful oxidants can add to aromatic rings to initiate O
2-mediated ring fragmentation, via well-established mechanisms in the gas-phase, that yield dicarbonyl products, such as glyoxal from benzene. Driving such ring fragmentation in aqueous phase holds potential for converting biogenic aqueous aromatic compounds into valuable dicarbonyl products with diverse chemical applications. Here, experimental kinetic measurements and density-functional theory (DFT)-based modeling are used to elucidate the mechanisms of ultrasound-driven reactions of benzyl alcohol. Moreover, benzyl alcohol reacts through alcohol oxidation, aromatic substitution, and ring fragmentation reactions, making it an ideal probe for ultrasound-driven reactions of aromatic compounds with diverse functional groups.
Ultrasound irradiation of aqueous benzyl alcohol formed aromatic compounds including benzaldehyde, phenol, and hydroxy-substituted benzyl alcohol. These same products were reported for gas-phase reactions seen in atmospheric chemistry. DFT calculations with implicit solvation showed that pathways in the gas-phase are also feasible in the ultrasound reactor. Oxalic acid, absent from gas-phase studies, was formed as primary product with large yields. DFT calculations showed that H2O facilitates oxalic acid formation by deprotonating •OOH to form •O2−, which undergoes nucleophilic attack of dicarbonyls mediating their oxidation to carboxylic acids. Such over-oxidation of dicarbonyls can therefore be avoided under acidic conditions that favor •O2− protonation to •OOH per the acid-base equilibrium. These combined computational and experimental insights establish the precedent that gas-phase radical mechanisms can inform the mechanisms of aqueous reactions occurring under ultrasonic irradiation. They elucidate the influential role of H2O as a protic solvent and offer strategies to mitigate its deleterious consequences to selectivity by controlling the pH.
Figure 1: Illustration of ultrasound-driven reactions benzyl alcohol in aqueous solution.