2024 AIChE Annual Meeting
(698e) Rational Design of Mixed Oxide Materials for CO2 Capture By Adsorption
Authors
In this work, we investigate comprehensively the use of a wide range of potential mixed oxide CO2 sorbents to be used in SEH2. The materials investigated include bimetallic hydrotalcites (HTs) with metal combinations such as Mg-Al, Cu-Al and Ni-Al, which are compatible with the reactions involved. The sorbents are synthesised via a co-precipitation method. Commercial solids are also assessed. The materials are fully characterised using several physicochemical techniques (e.g., BET, TGA, XRD, TEM, XPS, FTIR, ICP and crushing strength). State-of the-art equipment including an Intelligent Gravimetric Analyser is used to gain fundamental insights into reaction-adsorption performance. The experimental studies presented are carried out under realistic conditions. Full details are given elsewhere [3]. Dynamic models are developed and validated for the simulation of temperature-pressure swing operated reactors, using primarily gPROMS. Complementary equilibria and kinetic analyses are carried out using Matlab, Excel and Aspen Plus.
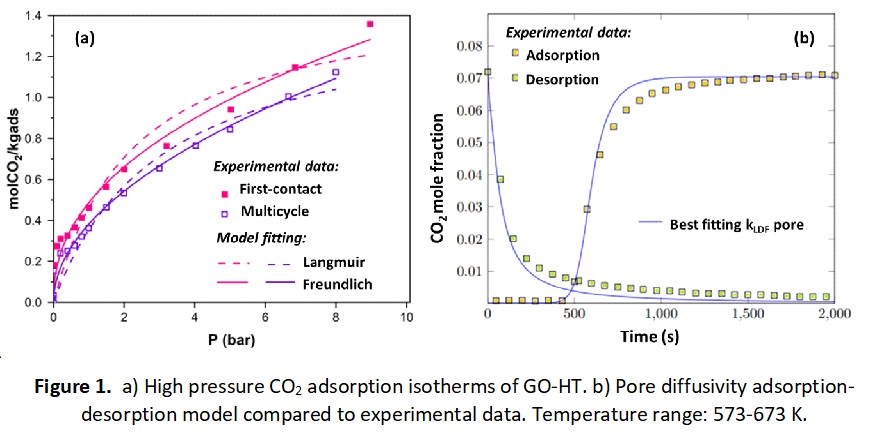
The results of this work show that the bimetallic HTs exhibit competitive CO2 adsorption capacities at 573 K and pressures up to 10 bar. However, the HTs show a decrease in capacity upon pressure and temperature swing cycling. Different functional groups to those of the fresh HTs are present in the samples exposed to the CO2 containing stream. In addition, the multicycle isotherms of HTs are not reversible showing the presence of a hysteresis loop. This can be correlated with the decrease in capacity observed. The fresh and cycled adsorption isotherms can be fitted by the Freundlich model. Alkali promotion increases their capacity whereas graphene oxide (GO) appears to enhance the multicycle-stability of HTs under the operating conditions investigated [4]. For instance, the initial capacity of the pure HT drops by 20-30% upon cycling while the uptake of the GO-HT hybrids is reduced only by 1-5%. The substantial benefits obtained by the presence of GO in HTs are attributed to electrostatic compatibility, excellent electrical conductivity, and enhanced mesoporosity and particle dispersion. The low loadings of GO, 5 wt%, needed in the hybrids benefit the design of compact and efficient sorption units.
The kinetics of mass transfer, along with the effect of particle porosity on the adsorption of CO2 on HTs in SEH2 are studied. A 1-D fixed bed reactor model based on a linear driving force description of the intraparticle mass transfer is developed. The model considers particle porosity and the non-linearity of the isotherms. Simulations show that a pore diffusivity model coupled with the Freundlich isotherm gives a good description of the CO2 adsorption on HTs. Competitive multicomponent (CO2-H2O) isotherms are suitable to simulate larger scale and high-pressure systems. Besides the CO2 adsorption analyses provided for HTs, analogous rational design studies are presented for MgO and FeO based materials, which are also promising CCS candidates [5]. The findings of this work constitute an important step towards the fundamental understanding and design of commercial adsorptive-reactive units particularly for low-carbon H2 production.
References
[1] M. E. Boot-Handford, P. Fennell et al. Energy Environ. Sci. 7 (2014), 130-189.
[2] D. Iruretagoyena, K. Hellgardt, D. Chadwick, Int. J. Hydrog. Energy, 43 (2018), 4211-4222.
[3] D. Iruretagoyena, P. Fennell, R. Pini, Chem. Eng. J. Adv., 13 (2023), 100437-100447.
[4] R. Menzel, D. Iruretagoyena, et al. Adv. Funct. Mater., 30, (2020), 2002788-2002802.
[5] J. Wong, D. Iruretagoyena, N. Shah, P. Fennell, Proc. R. Soc. A, 479 (2023), 20230173-20230195.
Acknowledgements
D. I. thanks Aida Gutierrez-Alejandre for discussions. SNI CONACYT, PAIP and ICRF are acknowledged for funding support.