2024 AIChE Annual Meeting
(696a) Numerical Analysis of Industrial Bioreactors to Accelerate Tech Transfer
Authors
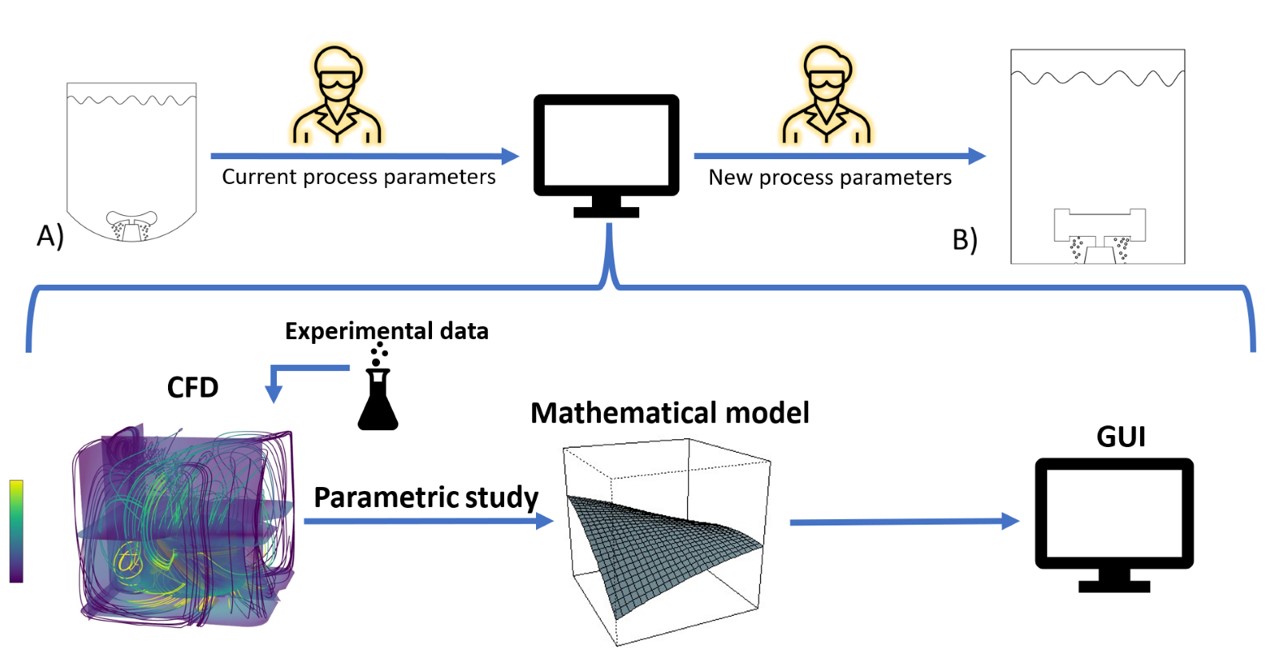
The workflow consists of development of a Computational Fluid Dynamics (CFD) model for each bioreactor system, which is verified and used to complete a parametric study. The parametric study employs a design of experiments (DOE) approach to obtain data for the creation of mathematical models, enabling tech transfer and scale-up between bioreactors. This mathematical model is implemented in a graphical user interface (GUI), providing an easy-to-use application.
In the current initiative, five single-use bioreactors and two glass bioreactors are included: Cytiva Allegro STR 200, Cytiva Allegro STR 1000, Cytiva Xcellerex XDR 200, Cytiva Xcellerex XDR 2000, Sartorius Biostat STR 200, Sartorius Univessel Glass 2 L, Sartorius Univessel Glass 10 L. To accommodate the cellular oxygen demand, bioreactor operations must sustain a sufficient oxygen transfer rate (OTR). The OTR is dependent on the volumetric mass transfer coefficient kLa , which can be calculated using Higgbie’s penetration theory:
kLa = C√DL(∈/υ)0.25a
Where DL is the diffusion coefficient of a gas species in the liquid, ∈ is the turbulence dissipation rate, υ is kinematic viscosity, and is interfacial area concentration, which can be calculated as:
a=6α/d32
Where α is gas volume fraction and d32 is Sauter-mean diameter.
CFD models were developed to predict hydrodynamic behavior during cell cultivation in the mentioned bioreactors. The goal for the models was to reach a combination of required prediction accuracy and solution time. The CFD modeling was performed with Ansys Fluent R2021 software. The realizable k-∈ model for turbulence and the Eulerian multiphase model were employed. The interfacial area was modeled using the interfacial area concentration approach with breakage and coalescence kernels. The rotation of the impeller was modeled using the multiple reference frame approach, and the case was solved as steady. The CFD model predictions were compared to the experimental data with mean relative error in the kLa values less than 15%. The P/V predictions have low variance; however, a bias was present due to different experimental setups, where the measurements were performed without gas sparging. The average solution time was approximately 1 day.
The CFD models were then used to perform a parametric study. The design of the experiment approach was selected, with three factors – working volume, agitation rate and gas flow rate studied at three levels (i.e., -50%, center-point, +50%). The center-point represents typical cultivation conditions used. The face-centered central composite design with 15 study points per bioreactor was selected to reduce the computational time without significantly diminishing information relative to the full-factorial DOE. The mathematical models for required hydrodynamic parameters were created using the locally estimated scatterplot smoothing technique.
The Shiny package in RStudio was used to develop an easy-to-use application. Scale-up and tech transfer are accomplished by fixing the oxygen transfer rate and a second parameter, which can be constant vvm, constant P/V, target agitation rate, target flow rate, hydrodynamic stress levels, or other additional terms. The application will be leveraged by process development and manufacturing experts to accelerate bioreactor scaling during tech transfer, reducing the need for resource-demanding wet-lab experiments.
Moreover, the CFD models developed in this work will be part of the “CFD library” for distribution within our R&D and manufacturing network. It is a library of all available CFD models for the equipment used, aiming to accelerate and improve the development of CFD models for specific purposes. Expanding this library to incorporate new vessels will enable more organizations and geographies to apply CFD to identify optimal operating parameters, perform facility fit evaluations, and even troubleshoot during runs.
References:
[1] M. Senior, Fresh from the biotech pipeline: record-breaking FDA approvals, Nature Biotechnology, 42 (2024) 355-361.
[2] S.M. Paul, D.S. Mytelka, C.T. Dunwiddie, C.C. Persinger, B.H. Munos, S.R. Lindborg, A.L. Schacht, How to improve R&D productivity: the pharmaceutical industry's grand challenge, Nature Reviews Drug Discovery, 9 (2010) 203-214.
[3] S.S. Farid, M. Baron, C. Stamatis, W. Nie, J. Coffman, Benchmarking biopharmaceutical process development and manufacturing cost contributions to R&D, mAbs, 12 (2020) 1754999
Figure 1 The operator inputs current process settings from the established process running on bioreactor A into the app. The app provides the operator with the input settings for the target vessel to maintain equivalent cultivation conditions while accepting user defined constraints.