Introduction
A promising category of catalysts for a wide range of organic reactions and gas separation applications is represented by Zeolitic frameworks loaded with diverse metal sites1. However, there are significant challenges to address, including diffusion limitations, catalyst lifetime, instability under harsh reaction conditions, and poisoning due to byproducts2. Herein, we study the desilication behavior of the MFI-zeolite framework using alkaline reagents, and concomitantly design a series of ethylenediamine assisted pathways to facilitate the selective deposition of metal clusters (Pd and Ag) in the zeolite. We attempted to design pathways to control and facilitate the incorporation of metal nanoparticles and clusters (Pd and Ag) on the selective topological locations of S-1 and ZSM-5. The metal incorporation in S-1 and ZSM-5 was carried out using three disparate pathways, namely, in-situ, single-step, and two-step routes.
Materials and Methods
To obtain ZSM-5 and Silicalite-1 crystals, silica and alumina sources (in case of ZSM-5) and a silica source (in case of Silicalite-1) were combined with an alkaline structure directing agent (SDA) were stirred at room temperature, resulting in the formation of a viscous gel. The gel was then subjected to hydrothermal treatment within the temperature range of 100 to 200°C for a duration of 24 to 96 hours. This process yielded the desired ZSM-5 and Silicalite-1 crystals. Subsequently, the obtained product underwent thermal treatment in an aqueous alkaline solution to achieve hollow zeolite crystals. To incorporate metal clusters and nanoparticles into the core-shell structure of ZSM-5 and Silicalite-1, the metal complex was reacted with zeolite, enabling the integration of the metal clusters within the core-shell zeolite.
Results and Discussion
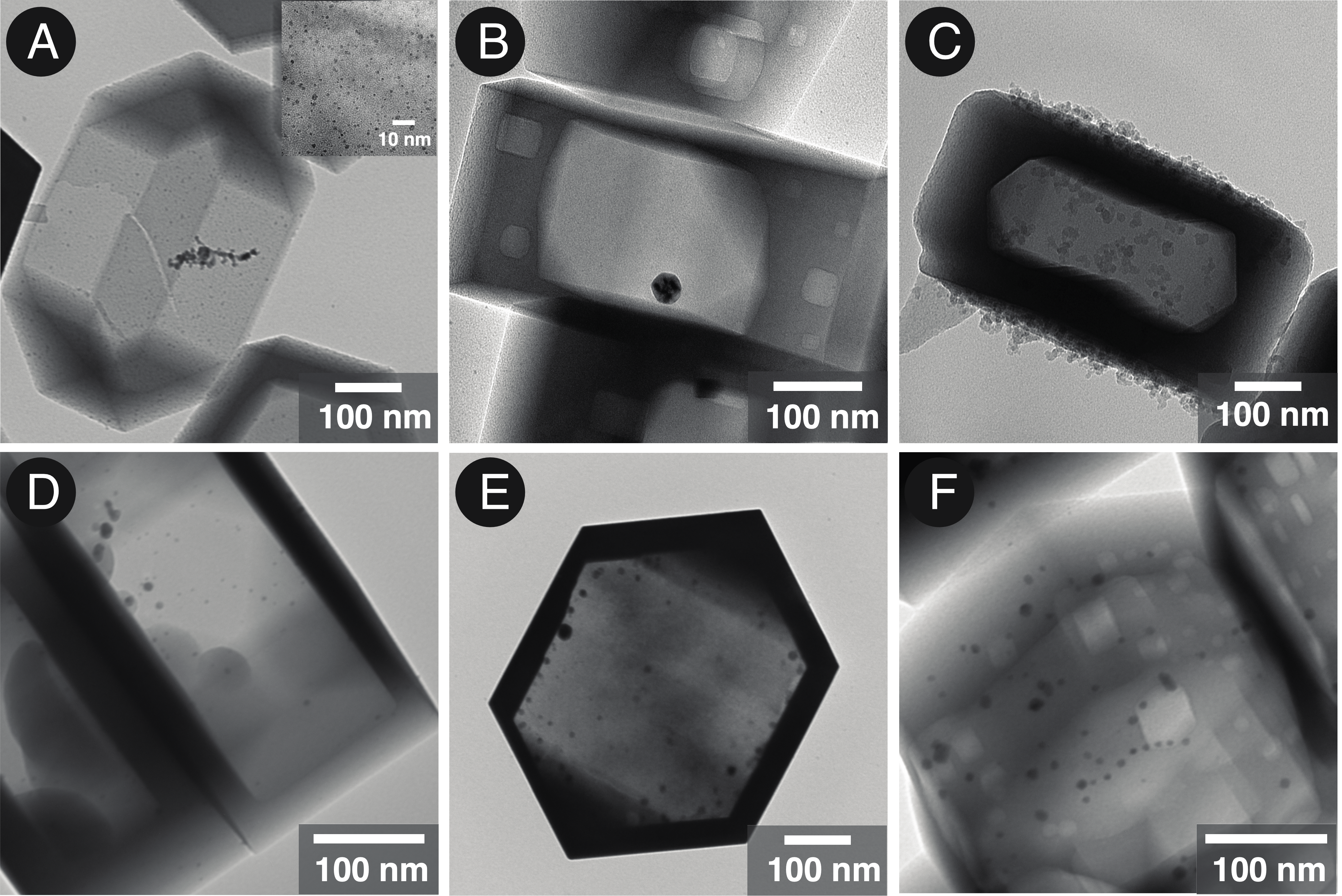
Treatment of MFI zeolite crystals with alkaline solution results in the formation of core-shell crystals, where the thickness of the shell ranges from 10 to 100 nm. For enhanced catalysis and stability, synthesizing core-shell zeolite crystals is highly desirable. We explored the new synthetic protocols here for obtaining MFI zeolite hollow crystals incorporated with metal clusters of Pd and Ag. Here, figure 1 (A), (B), (D) and (E) show the hollow zeolite crystals with metal nanoparticles of size 2 to 10 nm inside the hollow domain. Whereas, figure (C) and (F) shows that the small nanoparticles of the metal are dispersed on the surface of zeolite crystal. Similar trends are observed in the both the metals. We carried out three synthetic protocols to generate the zeolite crystals with controlled topological distribution of metal sites. The mechanistic studies and the detailed analysis results will be presented at the conference.
Significance
Active metal species incorporated into the MFI zeolite facilitate efficient heterogeneous catalysis in various important reactions by adjusting the zeolite's pore structures, acidic and basic properties, crystal sizes and morphologies. Our aim is to Extend the use of metal-in-zeolite catalysts from model reactions to various industrial processes, involving active metal sites and zeolite properties like acidity, basicity, and shape selectivity.
References
- Sun Q., et al., Advnced materials, 2021, 33, 51, 2104442
- Weckhuysen B. M. Chem. Soc. Rev. 2015, 44, 7022