Breadcrumb
- Home
- Publications
- Proceedings
- 2024 AIChE Annual Meeting
- Food, Pharmaceutical & Bioengineering Division
- Engineering Protein Therapeutics
- (685b) Engineering Novel Dual Decoy Receptor Antagonists for Neovascular Eye Diseases

Materials and Methods: VEGF-A and VEGF-C dimers as well as dual decoy receptor antagonists were produced in a HEK293F mammalian cell expression system and purified using Ni-NTA or Protein G resin before undergoing size-exclusion chromatography on an FPLC instrument. Bio-layer interferometry (BLI) binding studies were performed using an OctetRED96 instrument. Biotinylated VEGF-A or VEGF-C was immobilized on streptavidin-coated biosensors, and these sensors were then dipped into wells containing dilutions of antagonist or antagonist plus preincubated VEGF ligand for simultaneous binding studies. Signaling studies were conducted via western blotting of human microvascular endothelial cells (HMECs), and proliferation and migration analyses were performed using the xCelligence real-time cell analysis platform.
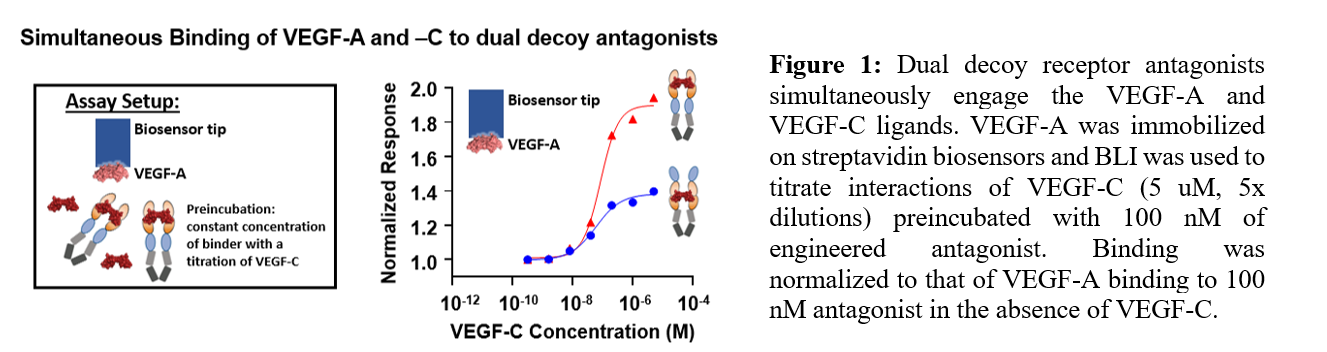
Results and Discussion: Dual decoy receptor antagonists were found to express well without significant aggregation in the HEK293F mammalian cell expression system. BLI studies demonstrated that these antagonists bound VEGF-A, VEGF-C, and PlGF-1 with high affinity, and also could simultaneously engage both VEGF-A and VEGF-C ligands (Figure 1). In vitro functional characterization studies show that engineered antagonists are able to inhibit VEGF-A and VEGF-C-based angiogenic effects both individually and in mixtures of the two ligands. In vivo studies are also currently underway investigating gene delivery of our antagonists as a strategy to block angiogenesis to treat neovascular eye diseases.
Conclusions: We have designed and characterized high-affinity protein antagonists that can engage both VEGF-A and VEGF-C simultaneously. These proteins have shown strong efficacy in inhibiting VEGF-driven angiogenesis through both in vitro signaling and various functional assays, and are being further characterized for in vivo efficacy with the goal of further developing these molecules as translational therapeutics to treat neovascular eye diseases.