Adsorptive desulfurization (ADS) has emerged as a promising alternative to conventional hydrodesulfurization, offering the prospect of producing sulfur-free fuels under ambient conditions with lower energy demands. Previous research has focused on ADS using zeolite sorbents with ion-exchanged cations to selectively adsorb high capacities of sulfur compounds from fuels.
1 Various surface metal species have demonstrated the ability to improve sulfur adsorption by providing available high-affinity active sites.
2,3 However, the available surface area of zeolites, in particular Y at 640 m
2/g, is decreased by metal ion-exchange and limits the maximum desulfurization potential. To overcome this challenge, higher surface area activated carbon (AC) materials have been considered as sorbents for ADS. While commercially available from many feedstocks including coal and biomass, AC derived from food waste (FWAC) has the added benefits of abundance and low cost while repurposing material otherwise destined for incineration or a landfill. FWAC materials have previously been successfully used as sorbents in other applications such as removal of phenol from wastewater.
4
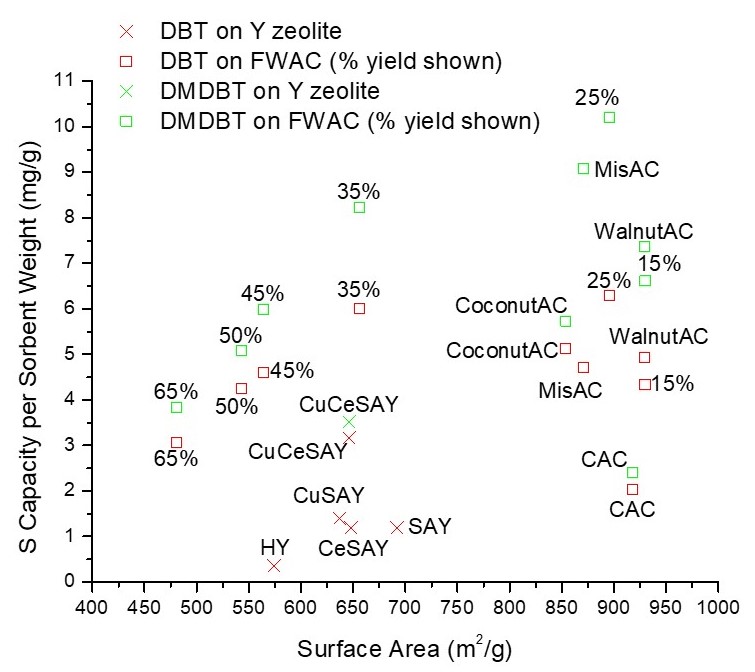
In this study we synthesized several FWAC materials via slow pyrolysis and subsequent physical activation by high temperature steam. By controlling the activation conditions, FWAC samples with a range of morphologies and characteristics were produced. These materials were characterized with various techniques to determine the physical and chemical properties and then tested for ADS using model fuels containing dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (DMDBT) in octane with added aromatic hydrocarbons. The optimal FWAC sample with a surface area of 896 m2/g was able to achieve maximum sulfur adsorption capacities of 6.3 mg S/g sorbent for DBT and 10.2 mg S/g sorbent for DMDBT. Characterization results indicate multiple mechanisms play a role is this excellent desulfurization. The synthesized FWAC samples show a linear relationship between micropore volume and sulfur capacity, suggesting physisorption of sulfur compounds in the micro pores of the carbon structure. In addition, ICP elemental analysis, XRD, FTIR, and TEM imaging show the presence of Ca, K, P, and Na species on FWAC. We hypothesize that these impurities from food waste provide suitable chemisorption sites for sulfur compounds. This is supported by results comparing FWAC to commercial AC and AC derived from other biomass sources including miscanthous, coconut shell, and walnut shell with similar surface areas. In these experiments the sulfur capacity of FWAC exceeded all other materials.
This application of FWAC represents a promising solution for reducing sulfur content in fuels, offering energy and cost savings compared to hydrodesulfurization. Moreover, the use of renewable materials as sorbents further minimizes the overall environmental impact of producing low sulfur fuels. The combination of impurities from food waste and tunable characteristics allow for high sulfur adsorption capacity exceeding other AC materials and zeolites. In addition, these attributes of FWAC may facilitate its use in various other adsorption and catalysis applications, transforming a waste resource into a valuable material.
(1) Lee, K. X.; Valla, J. A. Adsorptive Desulfurization of Liquid Hydrocarbons Using Zeolite-Based Sorbents: A Comprehensive Review. React. Chem. Eng. 2019, 4 (8), 1357–1386. https://doi.org/10.1039/c9re00036d.
(2) Lee, K. X.; Tsilomelekis, G.; Valla, J. A. Removal of Benzothiophene and Dibenzothiophene from Hydrocarbon Fuels Using CuCe Mesoporous Y Zeolites in the Presence of Aromatics. Appl. Catal. B Environ. 2018, 234, 130–142. https://doi.org/10.1016/j.apcatb.2018.04.022.
(3) Lee, K. X.; Crowl, T. B.; Sokol, H. J.; Morales-acosta, M. D.; Valla, J. A. Understanding the Role of Rare Earths in Zeolite Y on the Removal of Sulfur from Hydrocarbon Fuels. J. Phys. Chem. C 2021, 125 (17), 9107–9118. https://doi.org/10.1021/acs.jpcc.1c01103.
(4) Yu, L.; Gamliel, D. P.; Markunas, B.; Valla, J. A. A Promising Solution for Food Waste: Preparing Activated Carbons for Phenol Removal from Water Streams. ACS Omega 2021, 6 (13), 8870–8883. https://doi.org/10.1021/acsomega.0c06029.