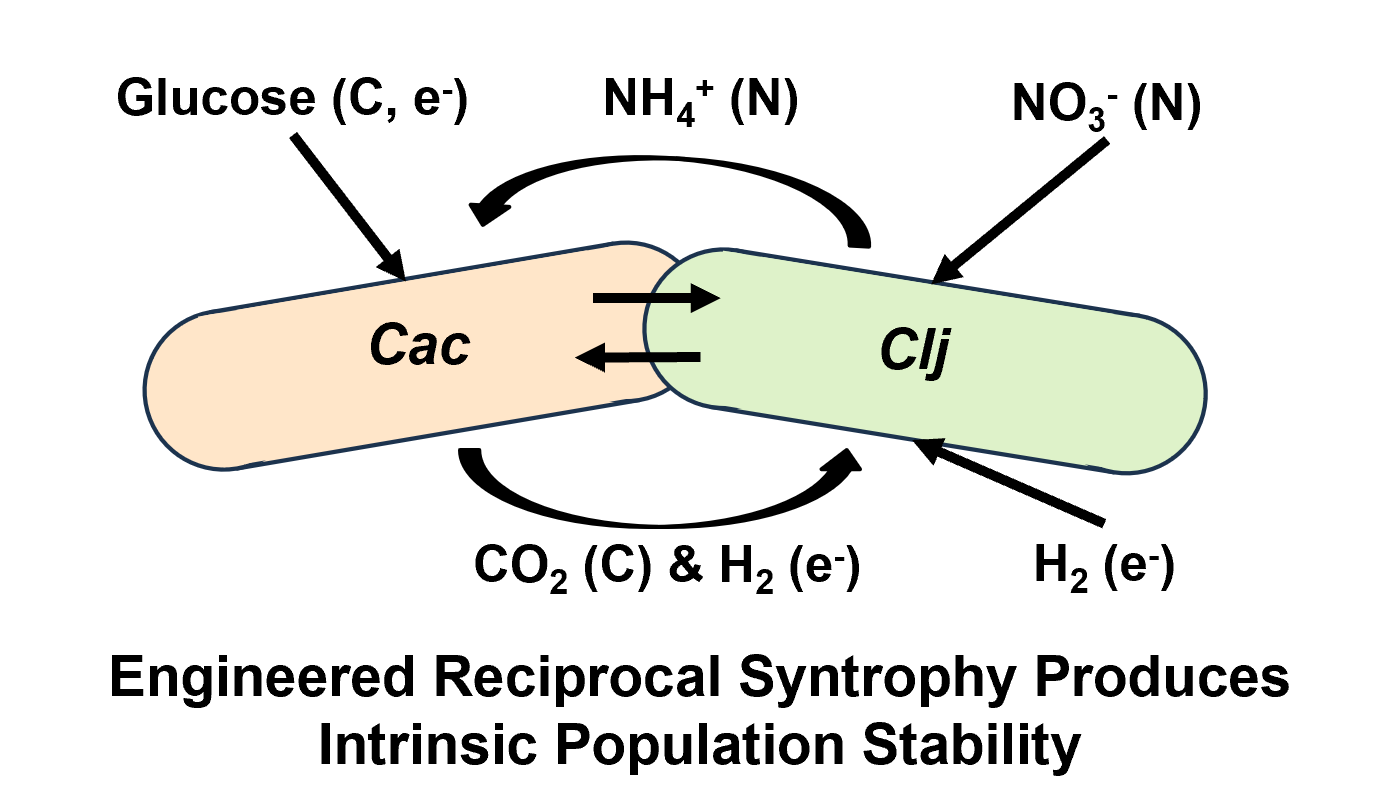
Although microbial biotechnology has traditionally relied on single microbes grown in isolation, cocultures can utilize the capabilities of multiple different bacteria, with little or no genetic engineering, to work together synergistically and accomplish tasks that would be impossible for any single species. In such a system, maintaining stable population ratios for repeatable performance is an important consideration. Recently, we have shown that coculturing
Clostridium acetobutylicum (
Cac) and
Clostridium ljungdahlii (
Clj) in a mixotrophic setting (using sugars, CO
2 and H
2 as substrates) increases sugar-substrate carbon and electron conversion via CO
2 and H
2 capture and synthesizes valuable products, such as isopropanol and 2,3-butanediol, that neither species can make independently. In this system growth of
Clj is constrained by
Cac, since
Clj relies on
Cac to convert glucose into CO
2 and H
2, which
Clj can use as carbon and electron sources, respectively. However, this mixotrophic syntrophy is unilateral;
Cac’s growth and performance is not constrained by
Clj. Consequently, population ratios between the two species can vary substantially throughout the course of fermentation and in different fermentation setups. Moreover, we have shown that population ratio is a crucial parameter for metabolite yields and productivity, and we hypothesized that population ratios may also influence heterologous cell fusion events we have documented in this pairing. Thus, rational control and stable maintenance of population ratio is important for both biotechnological applications and fundamental study of coculture systems.
In this study, we show that leveraging the different nitrogen utilization capabilities of these two organisms enables engineering of an intrinsically stable, bilateral mixotrophic syntrophy in which Clj relies on Cac for carbon and electrons and Cac relies on Clj for nitrogen. First, we show that since Clj, but not Cac, can convert nitrate into biologically useful ammonia, a minimal medium can be designed with nitrate as the sole nitrogen source such that Cac can only grow in the presence of Clj. Second, we show that feeding different rates of nitrate to cocultures in fed-batch mode produces different population ratios of Cac and Clj leading to different metabolic outcomes. Third, we utilize a chemostat to rationally maintain stable coculture ratios of Cac and Clj in continuous culture by feeding different ratios of glucose and nitrate. Finally, we explore the impact of this additional syntrophic layer of nitrogen cross-feeding on the heterologous cell fusion events we have previously observed between Cac and Clj.
Supported by the U.S. Department of Energy ARPA-E project under contract AR0001505.
N.B.W. and J.H. were supported in part by a U.S. Department of Education GAANN Fellowship under grant P200A210065.