Breadcrumb
- Home
- Publications
- Proceedings
- 2024 AIChE Annual Meeting
- Engineering Sciences and Fundamentals
- Fundamentals of Interfacial Phenomena I
- (678i) Inhibition of Ice Recrystallization By Regulating the Surface Free Energy of Nanoparticles

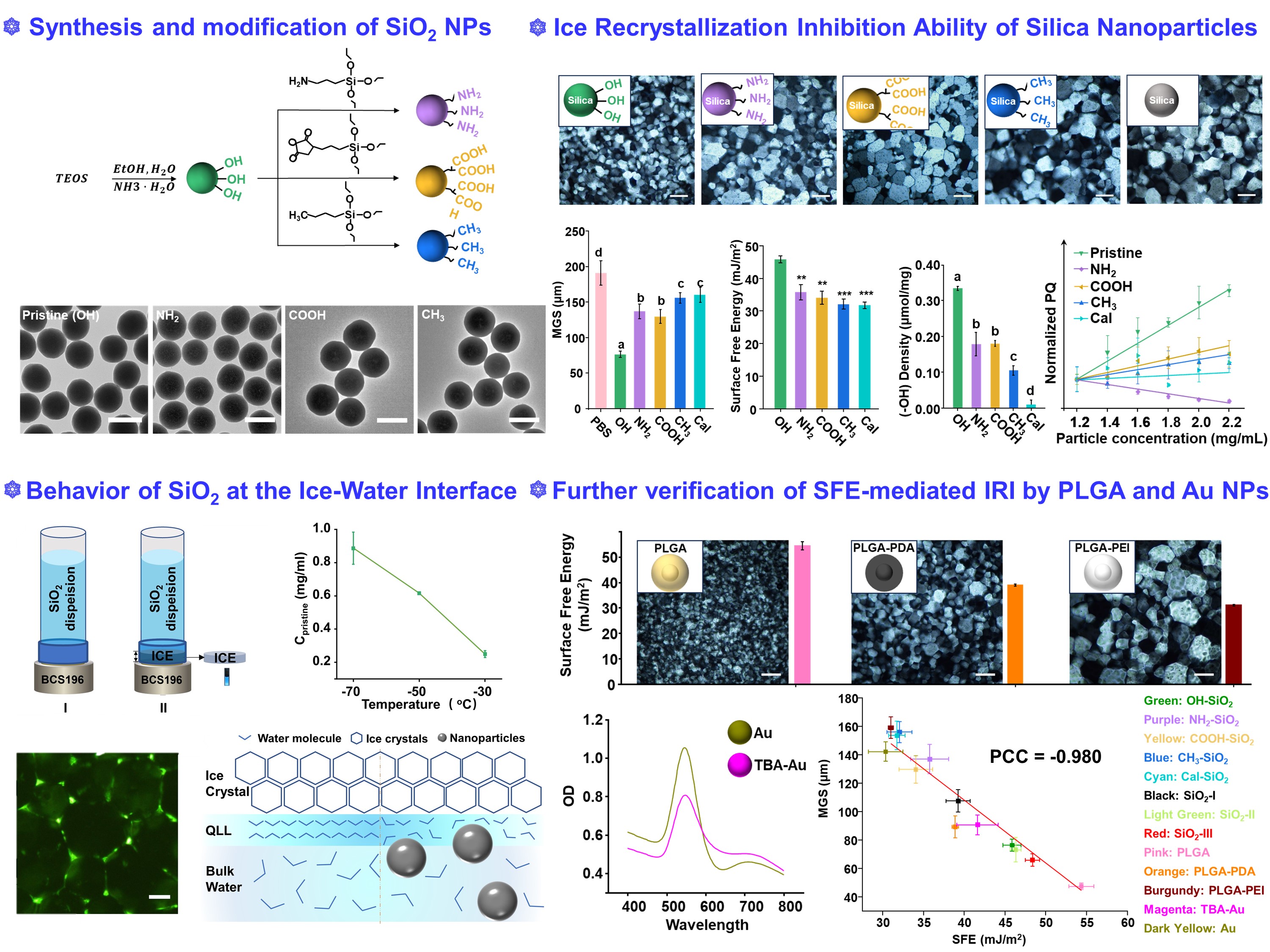
In this study, we established a comprehensive library of nanoparticles, including Stöber silica, poly(lactic-co-glycolic acid) (PLGA) and Au nanoparticles with tunable physicochemical properties, i.e., surface functionality, size, and composition. The pristine silica NPs were synthesized using a Stöber process, and the surface functionalization of silica NPs was performed using silane coupling agents. Furthermore, the densities of functional groups were quantitatively determined. It is worth noting that a library of particles with similar functional group densities (1µmol/m2) were obtained. Compared with pure PBS solution, the pristine Stöber silica NPs exhibited the smallest MGS, which was about 35% of the control group. In comparison, the IRI activities of amine-, carboxy-, and methyl-modified silica NPs were 70%, 67%, and 80% of the MGS of the control group, respectively. Polarized optical microscopy images showed that silica NPs with different surface groups exhibited various IRI activity.
In the presence of ice-control materials, the ice recrystallization is essentially mediated by the interaction between materials, liquid water and ice crystals. Thus, a modified ice affinity method was used to explore whether silica NPs could interact with liquid water or ice crystals. Different from nanomaterials such as GO and OQCNs that have adsorption equilibrium, it was found that while almost all of the silica NPs were kinetically trapped at a faster freezing rate, in our experimental settings, NPs were repelled by ice phase in a thermodynamic-driven process. The behavior of particles at the advancing solidification front can be explained by the net free-energy change (ΔFeng) of the system. The repulsion of nanoparticles suggested that ΔFeng was positive for the system after adding silica NPs during the recrystallization process. The distribution of nanoparticles was further confirmed. It was shown that particles were clearly distributed along the edge of the ice crystals during the recrystallization process, and further concentrated at the apex of the polygon-shaped ice crystals. Altogether, it can be proposed that when the thermodynamic process dominate the recrystallization, the quasi- liquid layer (QLL) with the ordered arrangement of water molecules around the ice crystals holds more nanoparticles than bulk water due to the rejection of nanoparticles, resulting in a higher disturbance of the QLL and hindering the transition of liquid water to the ice phase.
The inhibition of ice formation and growth could be ascribed to the chemical environments of the silica surface, and it has been demonstrated that the hydrogen bond formation between particles and water could hinder the ordered ice lattice to inhibit ice growth. Thus, the surface-active groups, i.e., silanols, on silica NPs that can form hydrogen bonds with water molecules were determined by acid-base titration. Additionally, the hydrophilicity of nanoparticles is a crucial physicochemical property that determines the interaction of particles with liquid water. Based on the fact that more hydrophilic surfaces are formed generally when the SFE of nanoparticles is higher, the SFE of NPs was determined using the maximum particle dispersion method. It can be found that the SFE of nanoparticles gradually increased in the order of Cal-SiO2 ≈ CH3-SiO2 < COOH-SiO2 ≈ NH2-SiO2 < Pristine-SiO2. The order of SFE and IRI activity of silica NPs was nearly identical. Generally, the liquid will preferentially spread on NPs with high SFE to reduce the energy of system according to the lowest energy principle. That is, in the system where materials, liquid and ice crystals co-exist, when the SFE between the materials and the liquid is higher, the disturbance of the QLL by the nanoparticles is stronger and ultimately induced stronger IRI activity.
Furthermore, silica NPs with different primary sizes and another nanomaterial library by using poly(lactic-co-glycolic acid) (PLGA) and Au were synthesized to verify the SFE-mediated IRI. When the libraries of Silica, PLGA and Au nanoparticles were combined, it was found that their SFE and IRI still showed a Pearson’s correlation coefficient of -0.980. This further suggests the role of SFE of nanoparticles in mediating the IRI activity. Generally, when the majority of nascent nuclei are composed of ice, these nanoparticles will be repelled by the ice phase and further accumulate at the ice-water interface. Therefore, it is highly possible that the behavior of ice growth and recrystallization are influenced by the surface characters of nanoparticles. Our experimental observation demonstrates that ice crystals growth is more strongly inhibited when the nanoparticles exhibit higher SFE in addition to the contributions from active groups capable of forming hydrogen bonds and hydrophilic surfaces. More liquid water tends to diffuse on their surfaces when nanoparticles show higher SFE, directly hindering the growth and recrystallization of ice. Additionally, we reported the effectiveness of Stöber silica and PLGA NPs in limiting ice recrystallization, which suggested that they may have the potential to be applied as ice-control materials such as cryoprotectants. Potentially, they can be used to reproduce the desirable properties of AF(G)Ps.
In conclusion, this study reveals the critical role of SFE of nanomaterial in mediating their IRI activity, which enables the prediction of IRI activity by the physicochemical properties of nanomaterials. Ice growth is spontaneous at low temperatures because of the abundance and ubiquity of interfaces. Future in-depth examination of ice growth and recrystallization behavior in the presence of non-ice solid material associated with the ice-water interface warrants further experimental and theoretical investigations that would greatly enhance our understanding of ice inhibition. Additionally, we provide a novel nanomaterial design strategy that relies on targeted regulation of physicochemical properties for the applications in cryoscience and cryotechnology.