2024 AIChE Annual Meeting
(655c) Development of a Solubility Measurement Workflow for Accelerating Crystallization Process Development for Pharmaceutical Compounds
Authors
A key aspect of developing a crystallization process involves the careful selection of solvents and antisolvents. Good solvents exhibit higher solvation capacity for process impurities than the active ingredient, thereby facilitating the purging of impurities and isolation of pure crystals. They also show a steep change in API solubility with temperature and thus maximize crystal yield with the lowest possible PMI. Similarly, effective antisolvents must mix well with the solvent and have minimal solubility for the API. Traditionally, solvent selection is conducted through a screening process, by manually testing several candidate solvents and antisolvents based on experience. This approach is labor, time, and material intensive and may yield sub-optimal solutions. Developing an efficient workflow for solvent screening and solubility measurement can thus accelerate crystallization development and lead to optimal processes with the above-mentioned characteristics. Several studies, in academia and industry, have explored the development of such workflows. These encompass the development of model-based tools to predict solubility ab-initio (Klajmon, 2022; Nordström & Rasmuson, 2009), automated high throughput platforms that can rapidly test several combinations of solvents (Shiri et al., 2021), and hybrid approaches combining these methods (Tan et al., 2019).
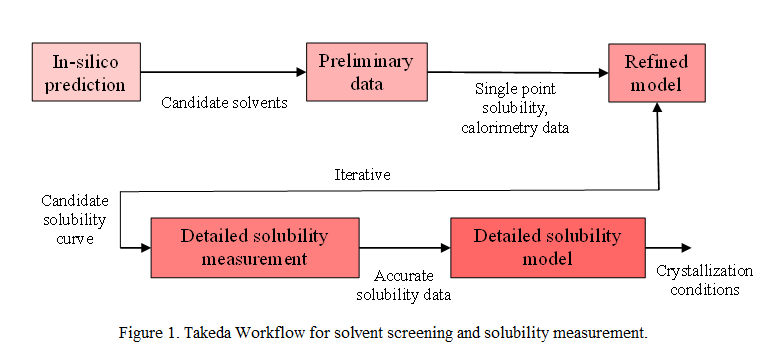
This study presents the development of a solvent screening and solubility measurement workflow for Takeda to support crystallization development for pipeline projects. This workflow is designed to minimize material usage, accelerate development timelines, and enhance overall process design. It will be hosted on an intra-group web platform and tailored to be user-friendly for chemists and engineers of all experience levels. The workflow will integrate model-based predictions, high throughput screening, and detailed experimental measurements for solubility (figure 1). At each step, multiple model packages and screening solutions will be used to ensure the workflow’s adaptability across different application areas and to facilitate the integration of latest technologies.
The first step in the workflow is to generate a list of feasible solvents and antisolvent for the API by performing a structure-based in-silico prediction of solubility. In the next step, API solubility is measured in these feasible solvents at room temperature. This data is used to refine the prediction made in the previous stage and generate a candidate solubility curve. Potential crystallization conditions are determined based on this solubility curve, such as solvent-antisolvent pairs, temperature ranges, expected yields and impurity purging. The scientist can then select the most suitable option among these and proceed with detailed solubility measurements. At this stage, experimental and predicted solubility can be compared, and further exploration can be carried out in case of substantial deviations. Finally, a detailed solubility model, specific to the system, is built through regression. This model can be used to determine crystallization conditions such as seeding point and can serve as a fast-executing model for process control.
The proposed workflow will be validated for three test APIs – two proprietary compounds and Paracetamol. The development of this workflow will thus create a systematic guide for solvent selection and solubility measurement and accelerate the development crystallization operations. Additionally, by taking into account factors impurity rejection, solvent toxicity, PMI, and product stability, it will facilitate the development of more optimal processes.
References
Brown, C. J., Mcglone, T., Yerdelen, S., Srirambhatla, V., Mabbott, F., Gurung, R., Briuglia, M. L., Ahmed, B., Polyzois, H., Mcginty, J., Perciballi, F., Fysikopoulos, D., Macfhionnghaile, P., Siddique, H., Raval, V., Harrington, T. S., Vassileiou, A. D., Robertson, M., Prasad, E., ... Florence, A. J. (2018). Enabling precision manufacturing of active pharmaceutical ingredients: workflow for seeded cooling continuous crystallisations. Molecular Systems Design and Engineering, 3(3). https://doi.org/10.1039/c7me00096k
Klajmon, M. (2022). Purely Predicting the Pharmaceutical Solubility: What to Expect from PC-SAFT and COSMO-RS? Molecular Pharmaceutics, 19(11). https://doi.org/10.1021/acs.molpharmaceut.2c00573
Nordström, F. L., & Rasmuson, Å. C. (2009). Prediction of solubility curves and melting properties of organic and pharmaceutical compounds. European Journal of Pharmaceutical Sciences, 36(2–3). https://doi.org/10.1016/j.ejps.2008.10.009
Shiri, P., Lai, V., Zepel, T., Griffin, D., Reifman, J., Clark, S., Grunert, S., Yunker, L. P. E., Steiner, S., Situ, H., Yang, F., Prieto, P. L., & Hein, J. E. (2021). Automated solubility screening platform using computer vision. IScience, 24(3). https://doi.org/10.1016/j.isci.2021.102176
Tan, J. S., Hilden, L. R., & Merritt, J. M. (2019). Applications of In Silico Solvent Screening and an Interactive Web-Based Portal for Pharmaceutical Crystallization Process Development. Journal of Pharmaceutical Sciences, 108(8). https://doi.org/10.1016/j.xphs.2019.03.013