Introduction
The increase in MP concentration in water ecosystems continues to pose a serious threat to aquatic ecosystems, and subsequently human health as a result of bioaccumulation 1. Significant number of MPs have been detected from different water matrices including surface water and wastewater treatment effluent, globally. Recent reports from South Africa have also indicated their presence in tap water in low concentrations 2. Water treatment plants globally, are not optimised or designed for the removal of MPs 3. A study by Kazour and team investigated the role of a municipal WWTP effluent as well as an abandoned coastal landfill as pathways for MPs insertion into the aquatic environment. They analysed the MPs in effluent, influent and sludge. They revealed that the WWTP exhibited a staggering daily discharge of ~ 227 million MPs 4. In another study, Gies and teammates investigated the retention of MPs in a WWTP in Vancouver, Canada. They revealed that a staggering ~1.76 trillion MPs per year enter the urban WWTP. They investigated the plants efficiency at retaining these MPs and to decipher how much of MPs aren’t retained and are released into the environment. Their study displayed that 99% of MPs that enter the WWTP had been retained. However, due to the extremely large volumes this major urban plant deals with, the 1% of MPs discharged into the environment equated to an astounding 30 billion MP particles being released into the sea 5. Therefore, technological innovations are required to alleviate the limitations embedded in these treatment systems 6. Nanotechnology has emerged as a pivotal technology to address a wide range of environmental challenges through performance enhancement 7. Therefore, in the present investigation, microplastics were removed from treated wastewater and drinking water via nanomaterials g-C3N4-Fe3O4 and BNNS- Fe3O4, separately.
Methodology
The nanomaterials (g-C3N4-Fe3O4, BNNS- Fe3O4) were synthesized by thermal polycondensation method via calcination followed by the in-situ incorporation of Fe3O4 through conventional co-precipitation using ammonium hydroxide solution. Synthesized nanomaterials were further characterised by Zeta potential, XRD, TGA, FTIR, BET, XPS, SEM-EDX, TEM and VSM. Various parameters (pH, time, nanomaterial dose, microplastic dose) were optimised through gradient experiments. The microplastics utilised for microplastic dose consisted of a combination of PE and PS (PE=125 and 180 uM > PS > 25 uM). All batch experiments were performed in 500 mL flasks containing ultrapure deionized Millipore water (18.2 MΩ cm), appropriate nanomaterial dose and MP dose. All experiments were conducted at 25 °C. Optimised operating parameters were applied in batch experiments to investigate g-C3N4-Fe3O4 and BNNS- Fe3O4 for the removal of different types and sizes of microplastics (PE(125 uM), PS(180 uM > PS > 25 uM), PE + PS (PE=125 and 180 uM > PS > 25 uM), PS (500 uM > PS > 180 uM), PP(3 mm), LDPE(5 mm), HDPE(5 mm)) from Millipore water, wastewater effluent and drinking water. A recyclability study was performed wherein each nanomaterial underwent 5 successive rounds of reuse employing optimum operating parameters in 500 mL flasks. To assess the toxicity of the nanomaterials and wastewater effluent, phytotoxicity experiments were conducted to determine seed germination indices (G.I. %) utilising 3 different crops viz. Vigna radiatus L. (moong), Cicer arietinum (chickpea) and Hordeum vulgare L. (barley)
Results and Discussion
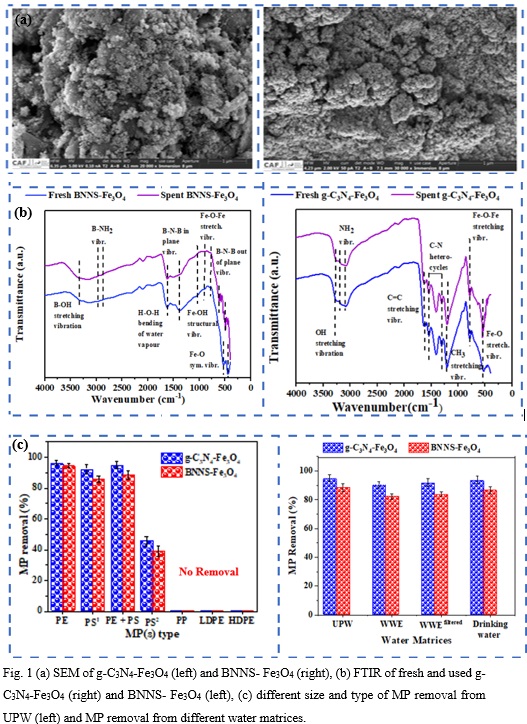
The maximum removal of PE (96.16%, size125 uM), PS (92.5% , size 180 uM > PS > 25 uM), PE + PS combo (94.89%, size PE=125 and 180 uM > PS > 25 uM), PS (45.62%, size 500 uM > PS > 180 uM), PP( 0%, size 3 mm), LDPE( 0%, size 5 mm) and HDPE (0%, size 5 mm) were noticed with catalyst g-C3N4-Fe3O4 under optimum operating conditions ([pH]= 4; time= 5 h; [nanomaterial]= 1.2 g/L; [MP]= 0.5 g/L) from UPW. The maximum removal efficiency of PE (94.44%, size 125 uM), PS( 85.96%, size 180 uM > PS > 25 uM), PE + PS combo ( 88.28% , size PE=125 and 180 uM > PS > 25 uM), PS (38.77%, size 500 uM > PS > 180 uM), PP(0 %, size 3 mm), LDPE(0 %, size 5 mm) and HDPE(0 %, size 5 mm) were observed with catalyst BNNS- Fe3O4 under optimum operating conditions ([pH] = 3; time= 12 h; [nanomaterial]= 0.9 g/L; [MP] = 0.5 g/L) from UPW (Figure 1). A direct correlation can be made between the removal rate and the size of the MPs. This is evidenced by the results that exhibit a decrease in removal rate as MP size increases. Due to the MPs size, in the case of PP, LDPE and HDPE, the magnet was unable to remove the MPs from solution. The investigation of nanoadsorbent removal efficiency in different water matrices yielded 93.7 and 86.56 % from drinking water via g-C3N4-Fe3O4 and BNNS- Fe3O4, respectively. A removal rate of 91.91 and 83.78 % was observed from domestic wastewater effluent filtered with a 0.22 µM filter for g-C3N4-Fe3O4 and BNNS- Fe3O4, respectively, whilst a removal rate of 90.28 and 82.23 % was observed from the same domestic wastewater effluent (unfiltered) for g-C3N4-Fe3O4 and BNNS- Fe3O4, respectively (Figure 1). The results for filtered and unfiltered wastewater effluent are similar indicating that filtering plays no significant role in improving the removal efficiency. The decrease in removal efficiency between the drinking water and the wastewater effluent could be attributed to various ions present in the wastewater effluent. It is possible that the existing ions in the wastewater significantly affect the performance of the adsorbent on MPs.
The reusability study revealed that both nanomaterials retained a removal efficiency of more than 50% after 5 cycles whilst g-C3N4-Fe3O4 retained a removal efficiency of almost 80% (79.74 %) after 3 cycles. The phytotoxicity of domestic wastewater influent, effluent, g-C3N4-Fe3O4, BNNS- Fe3O4, g-C3N4-Fe3O4 filtrate and BNNS- Fe3O4 filtrate were assessed through seed germination indices (G.I.%). Direct discharge of g-C3N4-Fe3O4 and BNNS- Fe3O4 in solution are toxic, however, the nanomaterial filtrate of g-C3N4-Fe3O4 and BNNS- Fe3O4 revealed mild toxicity (approaching non-toxic) and no toxicity, respectively. This indicates that filtering the nanomaterial solution not only ensures recovery of the nanomaterial but greatly diminishes the environmental impact of these NMs. It also indicates that leaching of the nanomaterials into the filtrate is minimal.
Key conclusions
The present study demonstrates that both BNNS-Fe3O4 and g-C3N4-Fe3O4 are effective removers of MPs from wastewater and drinking water. The reusability study demonstrates that both nanomaterials are highly stable and maintained satisfactory results after the fifth cycle. The phytotoxicity study exhibits that although direct discharge of nanomaterials in solution is toxic, the nanomaterial filtrate of g-C3N4-Fe3O4 and BNNS-Fe3O4 are mildly toxic (almost non-toxic) and non-toxic, respectively.
References:
- Dey, T. K.; Uddin, M.; Jamal, M., Detection and removal of microplastics in wastewater: evolution and impact. Environmental Science and Pollution Research 2021, 28 (14), 16925-16947.
- Bouwman, H.; Minnaar, K.; Bezuidenhout, C.; Verster, C., Microplastics in freshwater environments–a scoping study. Pretoria: Water Research Commission 2018, 3.
- Iyare, P. U.; Ouki, S. K.; Bond, T. J. E. S. W. R.; Technology, Microplastics removal in wastewater treatment plants: a critical review. 2020, 6 (10), 2664-2675.
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaa, S.; Khalaf, G.; Amara, R. J. M. P. B., Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. 2019, 146, 608-618.
- Gies, E. A.; LeNoble, J. L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E. R.; Ross, P. S., Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Marine Pollution Bulletin 2018, 133, 553-561.
- Hairom, N. H. H.; Soon, C. F.; Mohamed, R. M. S. R.; Morsin, M.; Zainal, N.; Nayan, N.; Zulkifli, C. Z.; Harun, N. H. J. E. T.; Innovation, A review of nanotechnological applications to detect and control surface water pollution. 2021, 24, 102032.
- Goh, P.; Kang, H.; Ismail, A.; Khor, W.; Quen, L.; Higgins, D., Nanomaterials for microplastic remediation from aquatic environment: Why nano matters? Chemosphere 2022, 134418.