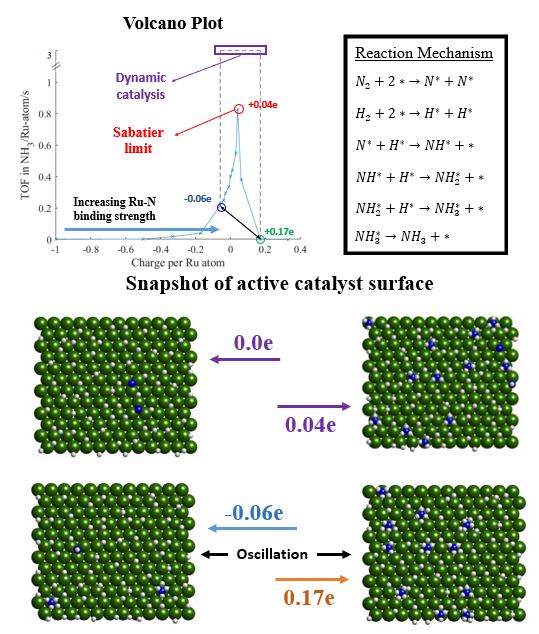
Ammonia is one of top chemicals produced with applications in agricultural and energy storage industry as a hydrogen carrier alternative. While catalyst development has significantly improved ammonia yield over the past few decades, further advances have been limited as the metals used is controlled by the Sabatier’s principle. Dynamic catalysis is a promising approach that may help to overcome this limitation. Recent efforts by Frisbie and Dauenhauer have reported on the development of “catalytic condensers” that enables facile change to the charge induced into supported catalysts
1,2,3. Herein, we evaluate the efficacy of dynamic catalysis using surface charge oscillation on Ru (0001) surface to enhance ammonia yield. We employ a combined Density Functional Theory (DFT) calculation together with Kinetic Monte Carlo (kMC) simulations to provide mechanistic insights into the role of surface charge oscillations in carrying out molecular transformations. DFT calculations were carried out as a function of charge as well as surface coverage to determine the kinetic parameters of the elementary steps and the binding energies of the adsorbates on Ru (0001) surface. These parameters were then subsequently used to parametrize lateral interaction models and provide elementary kinetic database used in kMC simulations to simulate turnover frequencies (TOF) as a function of reaction conditions and charge. kMC simulations on the static Ru (0001) surface revealed that the TOF decreased with increasing negative surface charge due to the weakening of Ru-N bond, which results in increased N
2 activation barrier. The TOF increased from 0.43 NH
3(Ru atom)
-1s
-1 on neutral Ru surface to 0.86 NH
3(Ru atom)
-1s
-1 at +0.04e/Ru atom surface charge due to stronger Ru-N bond strength with increasing positive surface charge. However, upon increasing the surface charge beyond +0.04e/Ru atom, the Ru-N bond strengthens leading to difficult desorption of NH
3. Therefore, +0.04e/Ru atom is the Sabatier limit with respect to the surface charges on the static Ru (0001) surface. kMC simulations on a dynamic Ru surface with square wave oscillation function between -0.06e and +0.17e per Ru atom yield a TOF of 2.97 NH
3(Ru atom)
-1s
-1, exceeding the Sabatier limit by approximately three times. Overall, the combination of DFT and kMC simulations helps to elucidate the effect of surface charge oscillation on ammonia synthesis reaction network and indicates that the Sabatier limit can be surpassed using dynamic catalysis and provides a direction for future experimental efforts.
References:
1Wang, Y., Udyavara, S., Neurock, M. and Frisbie, C.D., 2019. Field effect modulation of electrocatalytic hydrogen evolution at back-gated two-dimensional MoS2 electrodes. Nano letters, 19(9), pp.6118-6123.
2Onn, T.M., Gathmann, S.R., Guo, S., Solanki, S.P.S., Walton, A., Page, B.J., Rojas, G., Neurock, M., Grabow, L.C., Mkhoyan, K.A. and Abdelrahman, O.A., 2022. Platinum graphene catalytic condenser for millisecond programmable metal surfaces. Journal of the American Chemical Society, 144(48), pp.22113-22127.
3Onn, T.M., Gathmann, S.R., Wang, Y., Patel, R., Guo, S., Chen, H., Soeherman, J.K., Christopher, P., Rojas, G., Mkhoyan, K.A. and Neurock, M., 2022. Alumina graphene catalytic condenser for programmable solid acids. JACS Au, 2(5), pp.1123-1133.