2024 AIChE Annual Meeting
(627f) Structure and Activity of Single Cobalt Atoms Supported By Polymeric Carbon Nitrides
Authors
Qian Qian - Presenter, Worcester Polytechnic Institute
N. Aaron Deskins, Worcester Polytechnic Institute
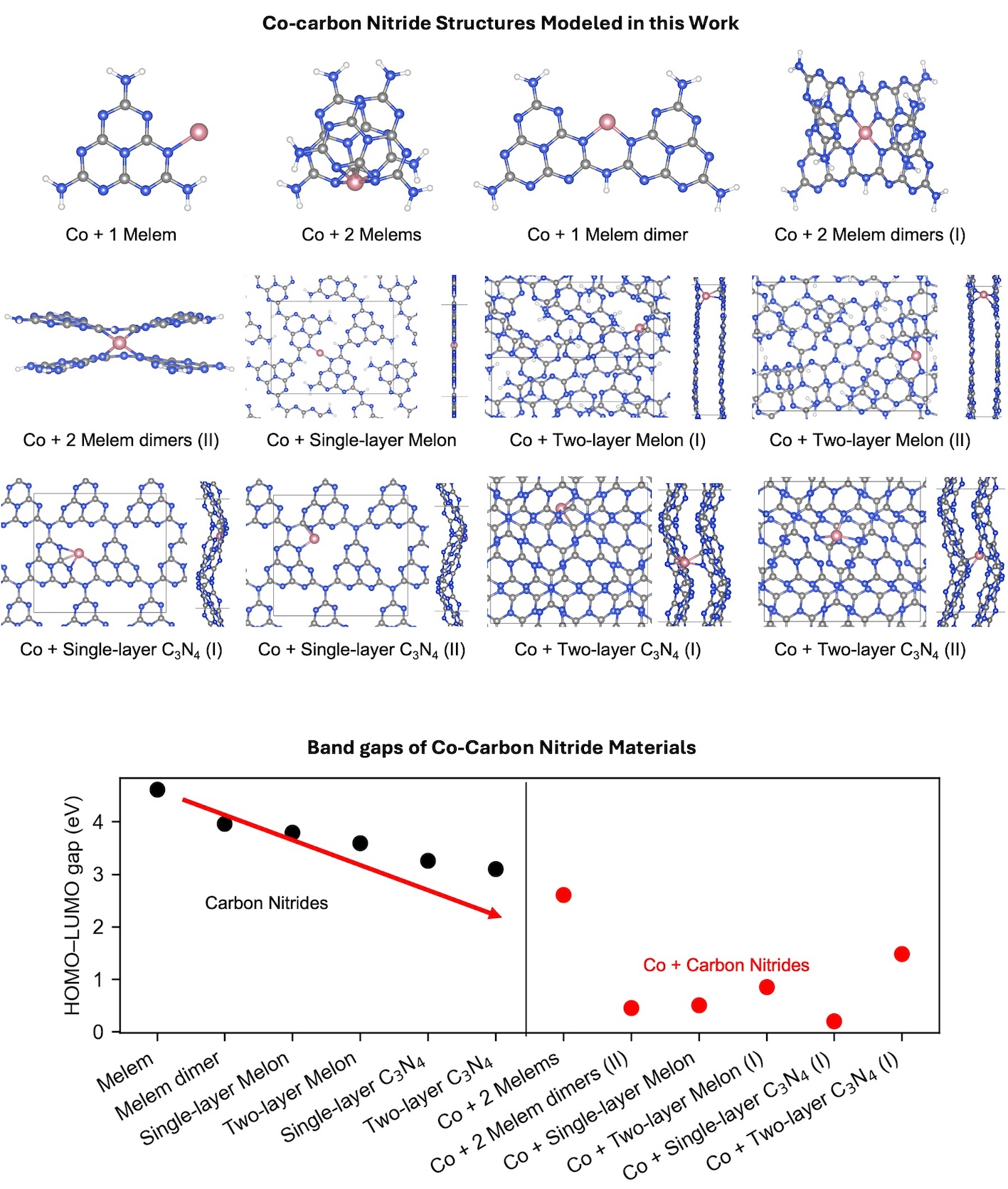
Two-dimensional carbon nitride materials have shown promise as supports for single-atom metals in a number of applications, including CO2 photoreduction. However, there is controversy on the structure of carbon nitride materials. Past literature suggests a polymeric structure is more likely to occur under typical synthesis conditions, and that previous literature has mischaracterized planar graphitic C3N4 as synthesized carbon nitride materials. Furthermore, the coordination number of the metal atom is a key factor in its catalytic properties, and literature suggests anywhere from 2 to 5 coordination numbers. In this work, we modeled 6 possible carbon nitride structures (as identified in the literature) using density functional theory. Co single atoms were modeled, as Co initiates CO2 adsorption and activation and has demonstrated significant selectivity towards CO in CO2 photoreduction. CO selectivity leads to reduced costs and effort to separate products. Our results show how the carbon nitride support structure affects the metal’s coordination number, as well as electronic and chemical properties of the catalysts.
We also modeled the formation of activated CO2, a critical step in CO2 reduction, over the various Co-carbon nitride structures. Our results indicate that CO2 could be activated on Co atoms in these Co-carbon nitride structures with binding energies of activated CO2 being -1 to -2 eV. We also analyzed how the Co cation’s charge and coordination number affect the CO2 activation and reactivity to show how CO2 reduction activity can be tuned depending on Co coordination and support choice.