In recent years, CO
2 utilization has garnered significant attention as a possible route for generating carbon-neutral/negative fuels and products. Currently, CO
2 utilization occurs in two steps, often in separate locations. As a result, this process requires energy-intensive compression and transportation to get the CO
2 from the capture site to the conversion site. Reactive carbon capture (RCC) is a promising alternative strategy that combines CO
2 capture and conversion into a single, integrated process. RCC can be further simplified by locating the CO
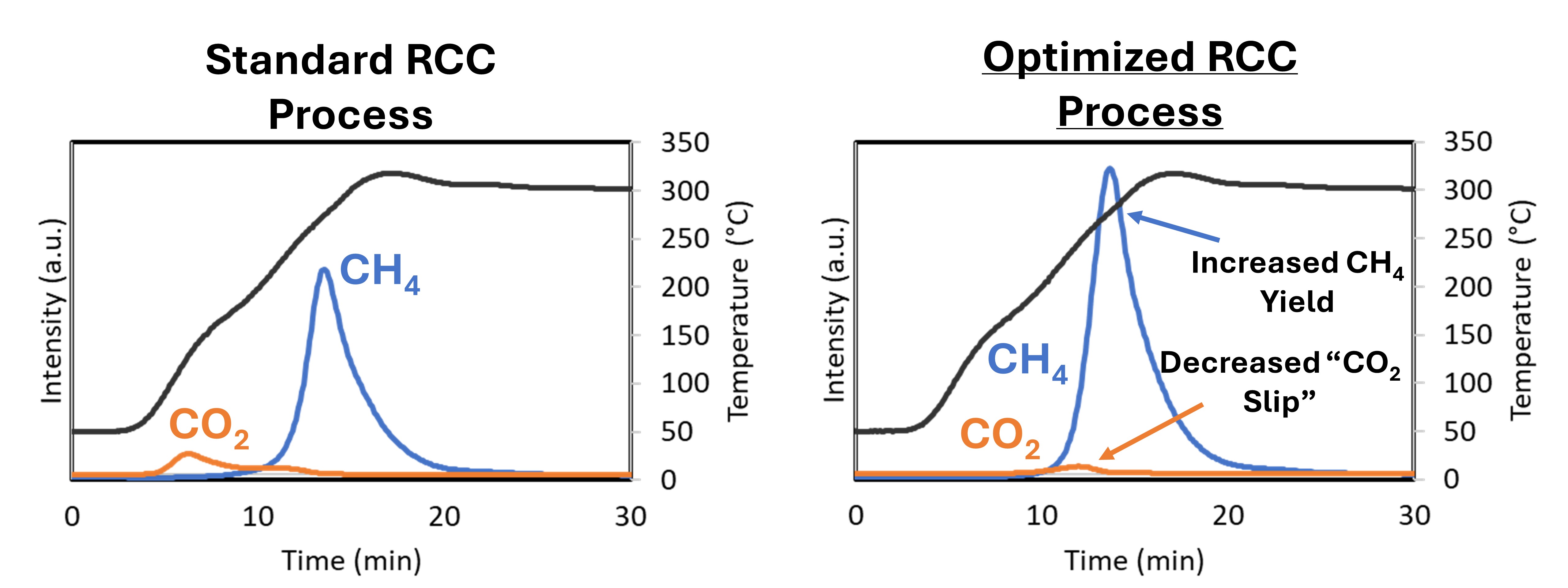
2 capture agent and catalytically active metal on the same material, known as a dual function material (DFM).
DFMs have been studied extensively for the capture and conversion of CO2 into CH4. Traditionally, the CO2 capture agent is an alkaline oxide (such as CaO, Na2O, or K2O), the conversion material is a catalytic metal (such as Ru or Ni), and the support is a high surface area oxide. The choice of capture agent and active metal has been studied in the literature extensively, but the role of the support is not well understood. In this presentation, we will compare the performance of DFMs supported on a variety of metal oxides (Al2O3, CeO2, SiO2, and TiO2) and discuss the impact of physicochemical properties on specific RCC metrics, such as CO2 conversion, CH4 production, and CO2 “slip” (desorbed, unreacted CO2). We will also report our findings on the impact of RCC process conditions (such as CO2 loading temperature, H2 concentration, CO2 concentration, rate of heating, and others) on overall RCC performance. Ultimately, we will show an optimized RCC process with rationally designed DFM that we are currently planning to scale-up using larger, continuous reactor configurations.