Whether the catalytic goal is to reduce oxygen content of biooils through hydrodeoxygenation or aqueous phase reforming of biorefinery waste streams to produce green hydrogen, the intricacies of adsorption and subsequent bond activations for biomass-derived oxygenated hydrocarbons deserve attention. Under reaction conditions, experiments have shown that high coverages on the catalyst surface are common, introducing intermolecular interactions that numerous low-coverage studies in computational heterogeneous catalysis may miss. The intermolecular interactions affect adsorption, reaction, and activation energies, reaction pathways, and ultimately, the potential energy surface (PES). The carboxylic acids were chosen as model compounds for our study because they contain functional groups found in other biooil constituents, yielding generalizable insights.
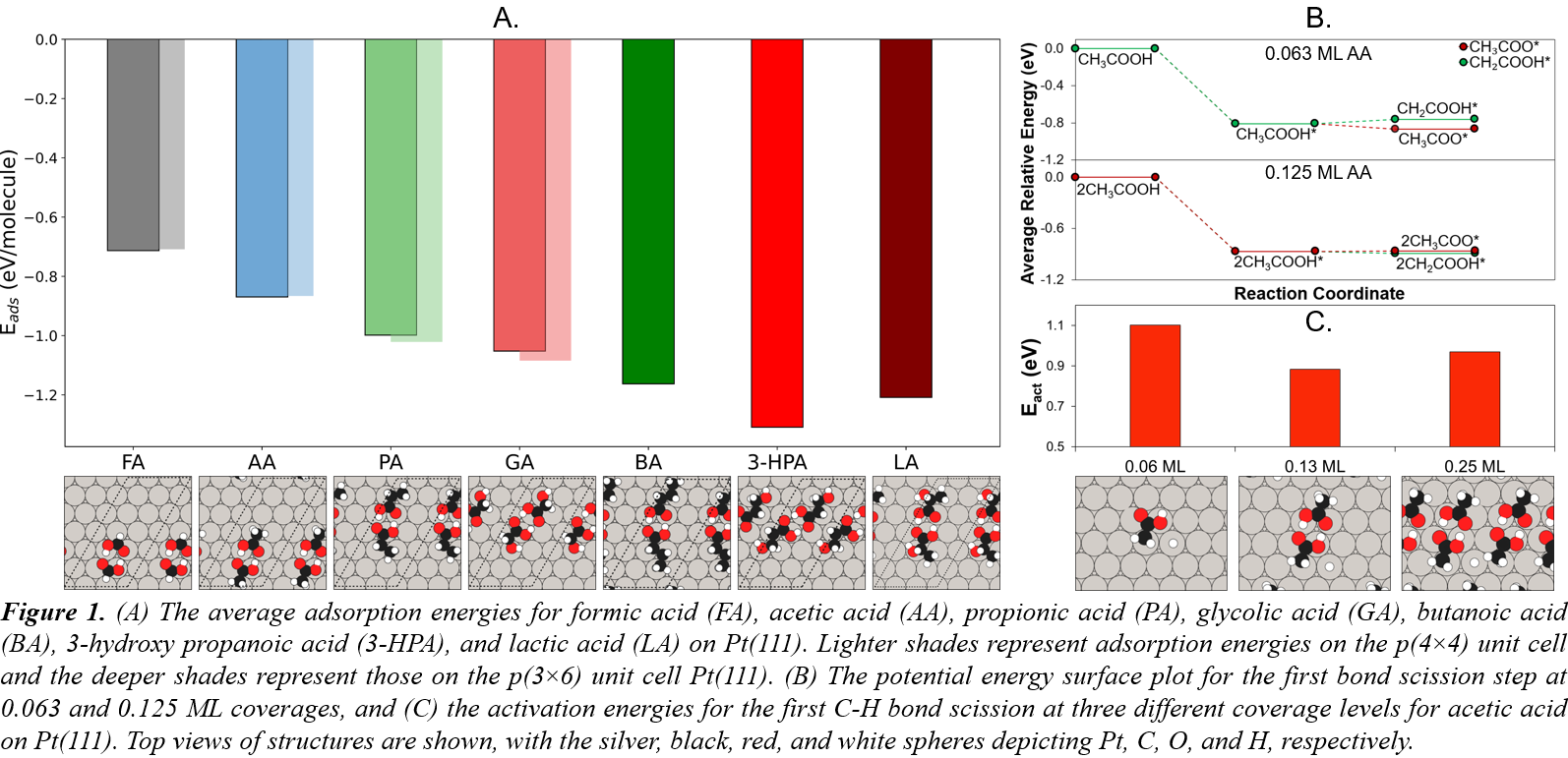
Utilizing density functional theory and ab initio molecular dynamics simulations, we explore the adsorption configurational space for seven carboxylic acids on Pt(111), Pd(111), and Ru(0001). We discovered that the most stable configurations maximize hydrogen bonding and the adsorbates whose structures allowed such interactions, adsorbed the strongest. Carboxylic acids form dimers, due to these attractive intermolecular interactions, which stabilize their adsorption on the catalyst surface at significant coverages (Figure 1). The low-coverage models fail to explain experimental findings that significant-coverage models adequately support. This highlights the importance of better understanding these dimers and their effects. Furthermore, using a database of adsorbed carboxylic acid dimers with varying functionalities and chain lengths, we developed an additivity rule for predicting their adsorption strengths. The effects of coverage and configuration on the PES were determined, e.g., for acetic acid, the reaction pathway shifts toward C-H scission as coverage increases, with the activation energies varying according to attractive/repulsive interactions. Altogether, our work highlights the catalytic impacts of these unexplored dimers. We demonstrate that accurate models of reactive interfaces require including such intermolecular attractions, and determine how dimer formation stabilizes reaction intermediates and affects overall reaction networks.