Breadcrumb
- Home
- Publications
- Proceedings
- 2024 AIChE Annual Meeting
- Process Development Division
- Poster Session: Process Development
- (572g) Methanol Synthesis Mini-Plant with Product Condensation and Reactant Recycle Operated with COx

For the reduction of CO2 emissions alternatives to fossil carbon sources are of increasing interest for chemical industry. Chemical utilization of carbon dioxide or carbon-containing waste streams offers a way to convert carbon into valuable base chemicals. One particular example is COx based methanol which can be utilized in various ways as an energy storage, clean fuel and building block for chemical industry.[1,2]
Experimental Setup
A mobile small-scale methanol mini-plant was realized, with the aim to study the methanol synthesis process from COx including a reactant/product separation through condensation, high-pressure gas recycles and purge as well as different connection possibility of two fixed bed reactors, the separation and recycle. Furthermore, the scale is this small that catalysts can be tested on a pre commercial production scale and lower amount that approx. 50 mL h-1 of liquid product mixture are produced, allowing detailed product characterization but avoiding huge amounts of storage and disposal. Through the small scale the mini-plant is also mobile and easy to transport to real H2 and COx sources. The composition of the streams before and after each reactor can be analyzed by a gas chromatograph which detects the permanent gases via TCD and the larger carbon compounds and oxygenates via FID. The feed stream for the reaction can be mixed from different pure gases (H2, CO2, CO, CH4 and N2) and from real COx sources like syngas from gasification processes in any ratios.
Results
First experiments were carried out with 30 g of commercially available CZA-catalyst (copper zinc oxide on aluminum oxide), a reaction temperature of 230 °C and a pressure of 30 bar. The stoichiometric feed stream of CO2 and H2 with 30 g h-1 was mixed from bottled gas. The results show that a full conversion of the reactants is possible when increasing the recycle ratio. At the same time a small accumulation of byproducts can be observed in the recycle stream. Nevertheless, no detectible purge stream was necessary. Moreover, the start-up process of the reaction including the recycle can be recorded. When increasing the reactor temperature from RT to 230 °C a first decrease of the off-gas mass flow can be observed at a reaction temperature of about 180 °C. Full conversion is reached at a specific combination of reaction temperature and recycle ratio for the given point of operation. Investigation of dynamic plant behavior shows a pressure dependence for the time until a new stationary point is reached.
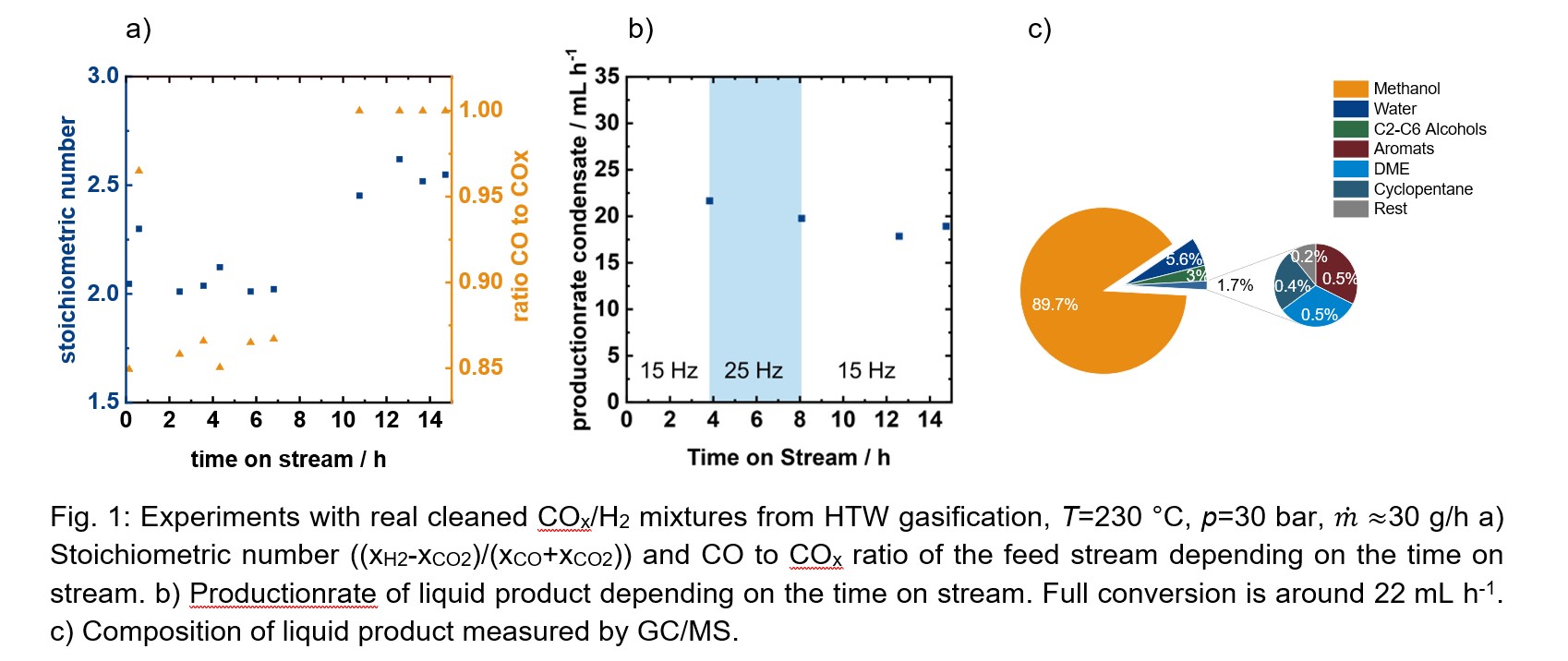
Experiments with real COx mixtures from a 500 kW Hochtemperatur-Winkler-gasification (HTW) and syngas postconditioning operated by the Institute for Energy Systems and Technology of TU Darmstadt (Prof. Epple) were carried out to investigate the influence of real syngas on the recycle ratio, the catalyst stability and the spectra and accumulation of byproducts. The results show that full conversion is possible despite a fluctuating feed composition (Fig.1 a and b) and the enrichment of inerts in the recycle stream can be handled. In the condensate byproducts of the gasification are traceable as well as higher alcohols formed as byproducts during methanol synthesis (Fig.1 c). Further experiments are planned with different operation conditions and feed mixtures of pure CO and CO2 investigating the plant behavior inside the recycle regarding recycle ratio, changing composition of reactants and byproduct formation.
[1] G. A. Olah, Beyond oil and gas. The methanol economy, 2. Aufl., Wiley-VCH, Weinheim, 2009.
[2] A. Goeppert et al. Chemical Society reviews 2014, 43, 7995.