2024 AIChE Annual Meeting
(569q) Influence of Zeolite Framework on the NOx Operating Cycle in Passive NOx Adsorbers
Authors
Divesh Bhatia - Presenter, Indian Institute of Technology Delhi
Tuhin Suvra Khan, Indian Institute of Technology Delhi
M. Ali Haider, Department of Chemical Eng., IIT Delhi
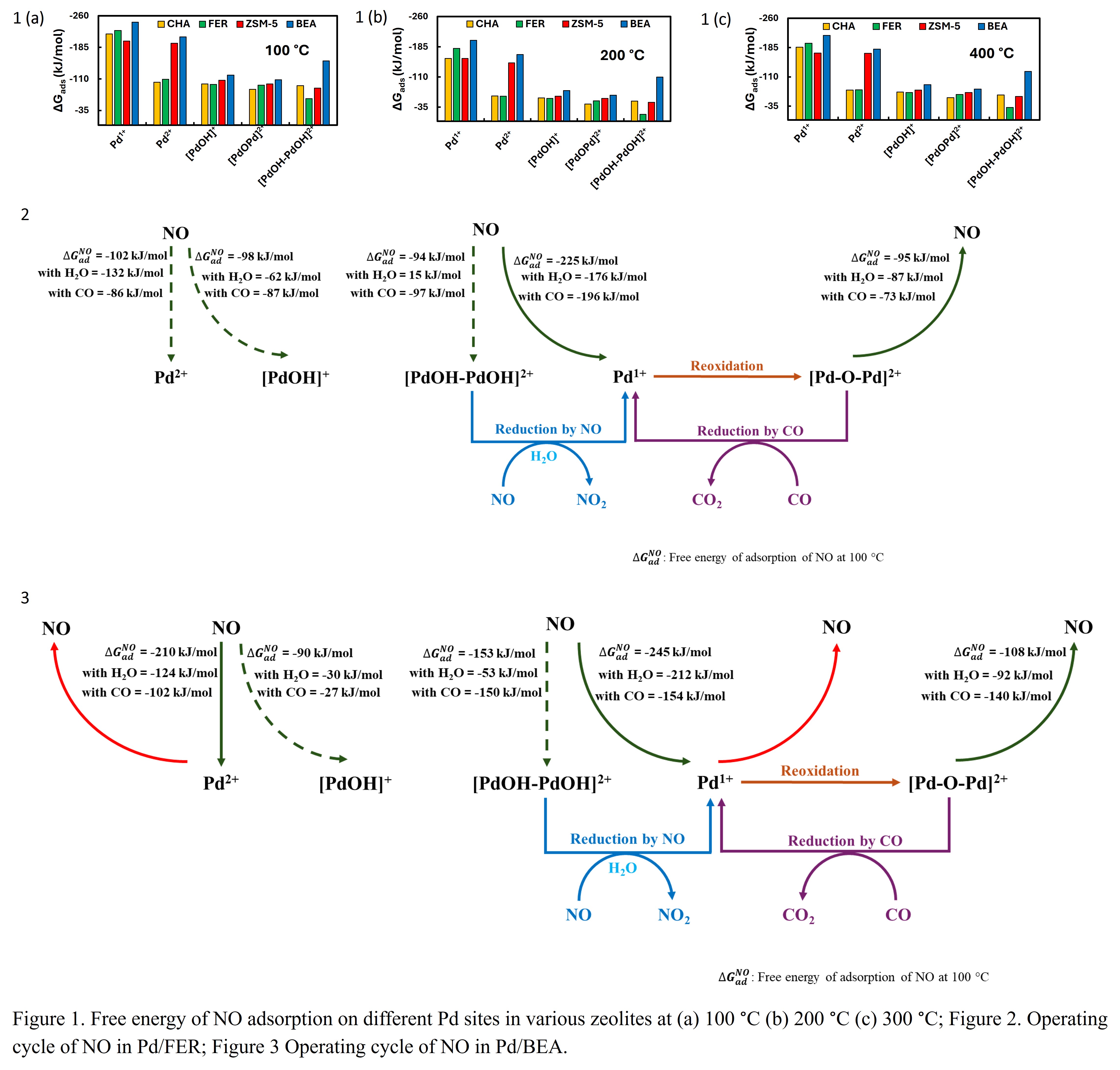
Pd/zeolites (Pd/BEA, Pd/CHA, and Pd/ZSM-5) are suitable materials for passive NOx adsorption due to the presence of multiple Pd sites such as Pd2+, Pd1+, [PdOH]+, [Pd-O-Pd]2+, and [PdOH-PdOH]2+ which are active for low temperature NOx storage. Utilizing density functional theory (DFT) simulations, the impact of zeolite framework on the mechanism of NO adsorption on different Pd/zeolites is studied. The analysis of free energy of NO adsorption across various Pd sites within different zeolites revealed a decrease in binding strength with increasing temperature (Figures 1 (a-c)). Irrespective of the zeolite framework, NO binds strongly on Pd1+ site even in the presence of H2O and CO, suggesting that Pd1+ is the optimum adsorption site in all zeolites. The binding strength of NO on Pd(II) sites is relatively weak due to which they are more suited for desorption. However, NO binding on Pd2+ and Pd1+ is similar in BEA and ZSM-5, which indicates that both Pd1+ and Pd2+ could act as adsorption sites whereas other Pd(II) species with relatively lower NO binding strength are the preferred desorption sites. Also, Pd/BEA exhibits the highest NO binding strength among all zeolites. Additionally, the free energy of activation for the interconversion of various Pd sites in the presence of NO, H2O, and CO is determined at 100 °C, revealing the significant influence of the zeolite framework on various transformations. The formation of Pd1+ site via the reduction of dimeric Pd(II) species is favorable in ZSM-5 whereas it exhibits high activation energy in CHA. These site-specific NO binding energy trends and the energetics of interconversion between sites result in differences in the NOx adsorption-desorption cycle which can predict the experimental trends over various zeolites. The proposed operating cycles of NO in Pd/FER and Pd/BEA are shown in Figures 2 and 3, respectively.