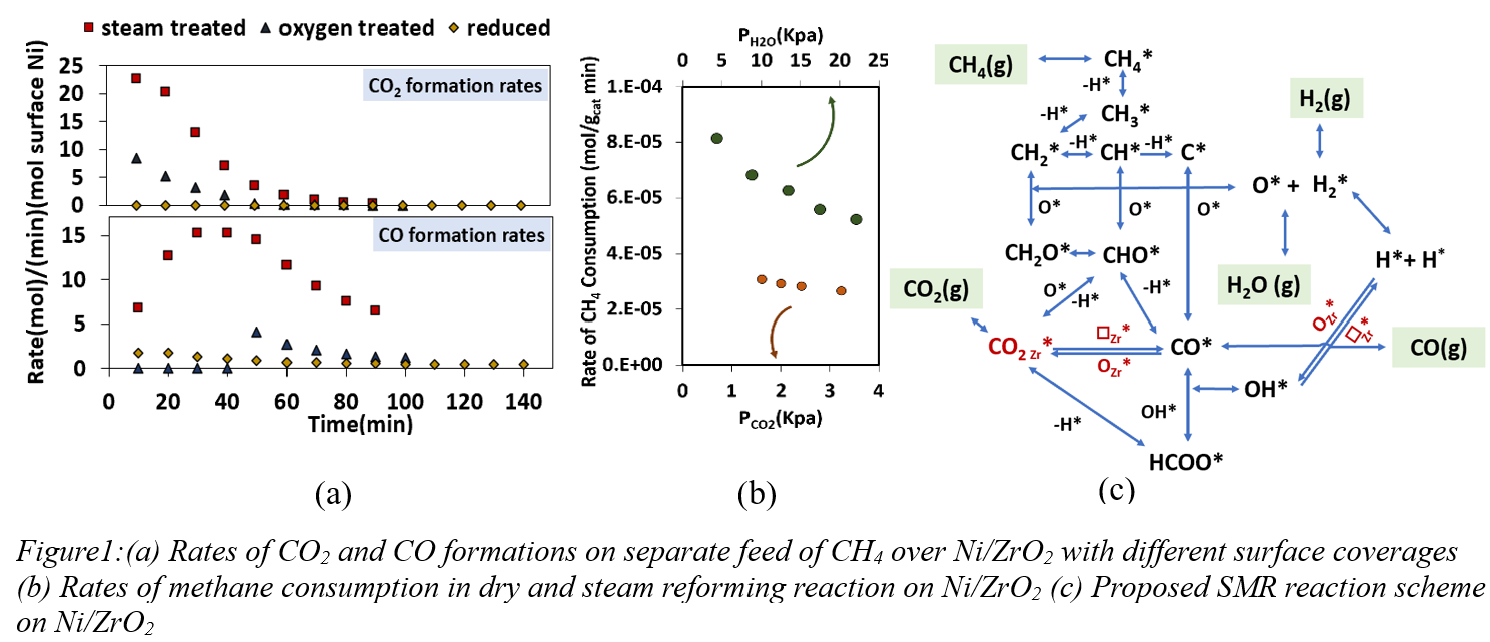
Steam methane reforming (SMR) remains the predominant industrial process for producing hydrogen, with its significance increasing to meet H
2 demand currently at 95 million tonnes per year. Accordingly, the development of efficient, cost-effective catalysts for SMR processes continues, relying on an understanding of kinetics and mechanisms mediating SMR catalysis. Support effects constitute key component of this understanding, with alumina and zirconia supports receiving extensive attention. ZrO
2, for instance, possesses acid-base centers and surface hydroxyls, increasing activity and reducing carbon build-up. In this work, we elucidate the role of key surface species such as hydroxyls and formates, and the effect of the ZrO
2 support in stabilizing these species. Figure-1(a) shows rates of formation of carbon oxides are higher when methane is introduced onto a Ni-ZrO
2 surface with hydroxyls (steam pre-treated samples) compared to an oxygen-covered one (oxygen pre-treated) or metallic nickel-containing surface (H
2 reduced). These data capture the role of hydroxyl species in enhancing SMR activity. Figure 1(b) shows methane consumption rates are similar despite a lower pressure of CO
2, pointing to a considerable inhibiting effect of CO
2. We show that this inhibiting effect can be attributed to oxygen vacancy defects that promote CO
2 adsorption at interfacial sites, leading to the formation of adsorbed CO* on Ni, thereby healing interfacial vacancies (Figure-1c). Significant coverages of formate intermediates resulting from surface hydroxyls and CO*
and quantified using in-situ infrared spectroscopy help explain the adverse effect of CO
2 feed concentration on methane conversion. A reaction mechanism involving interfacial hydroxyls, oxygen vacancies, and formate species (Figure-1c) was proposed and validated using a detailed kinetic model that accounts for each of the proposed steps. This study provides a quantitative description of the role of interfacial species in SMR chemistry and provides guidance on aspects of support and active metal in catalyst design for reforming reactions.