2024 AIChE Annual Meeting
(569fo) Mitigation of Methane from Low Concentration Sources Using Transition Metal Based Oxidation Catalysts
Authors
Methane (CH4), as a non-CO2 greenhouse gas in the atmosphere, poses a significant challenge to the global objective of limiting Earth's temperature rise to below 2°C. [1]. Despite the fact that methane is being emitted in smaller quantities than CO2 [2], its ability to trap heat in the atmosphere, known as its "global warming potential," is 28 times greater than that of CO2 on 100-year timescale [3]. Therefore, by catalytically converting CH4 to CO2, it is possible to avoid CO2 equivalents to the atmosphere [4].
Various methods for methane abatement have been studied, with noble metal-based (Pt/Pd) thermos-catalytic catalytic oxidation standing out as the most investigated technique [5]. However, elevated cost and susceptibility to poisoning have prompted investigations for alternative solutions. Transition metal oxide (TMO) catalysts have emerged as promising alternatives, given their abundance in the Earth's crust, cost-effectiveness, diverse oxidation states, and abundant acidic and basic sites [6]. While many TMO have demonstrated promising catalytic performance, their underlying reaction mechanisms have been relatively underexplored and remain subject to debate. This knowledge gap impedes the further optimization of TMO catalytic systems. Therefore, in this work, efforts are directed towards understanding the reaction mechanisms of TMO catalysts to facilitate their enhanced utilization in catalytic lean methane oxidation. Subsequently, various preparation methods are analyzed to investigate their impact on the catalyst's structure, morphology, and exposed crystal planes. Additionally, the synergistic effects of binary metal oxides mixtures are studied to obtain highly active and stable catalysts capable of accelerating methane combustion at low temperatures.
Methodology
In this study, different TMOs and mixtures thereof were synthesized, namely: Co3O4, NiO, CeO2 using various precursors. Different preparation methods such as coprecipitation, mechanochemical reaction and thermal decomposition were employed to prepare the catalysts, and their effects on catalytic activity are assessed.
X-ray diffraction (XRD) analysis is used to determine the crystal structures of the calcined samples while N2 physisorption is used to determine the textural properties of the prepared catalysts. The morphology and particle size distribution are assessed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Additionally, the surface oxidation state of the catalysts prior and after the reaction are analyzed via X-ray photoelectron spectroscopy (XPS).
The methane oxidation performance of various TMO catalysts is evaluated in a fixed-bed stainless steel reactor. The temperature ranges from 250 to 600°C, with a feed composition of 1 vol% CH4 in air at a space velocity of 18000 h-1 (NTP). The concentration of CH4 in the inlet and outlet gases is measured using an online flame ionization detector (FID). Catalyst activity is assessed based on CH4 conversion, calculated as (Cin−Cout)/Cin × 100%, where Cin and Cout represent the methane concentrations at the inlet and outlet, respectively. Activity data is recorded at each measurement point after stabilization.
Preliminary results:
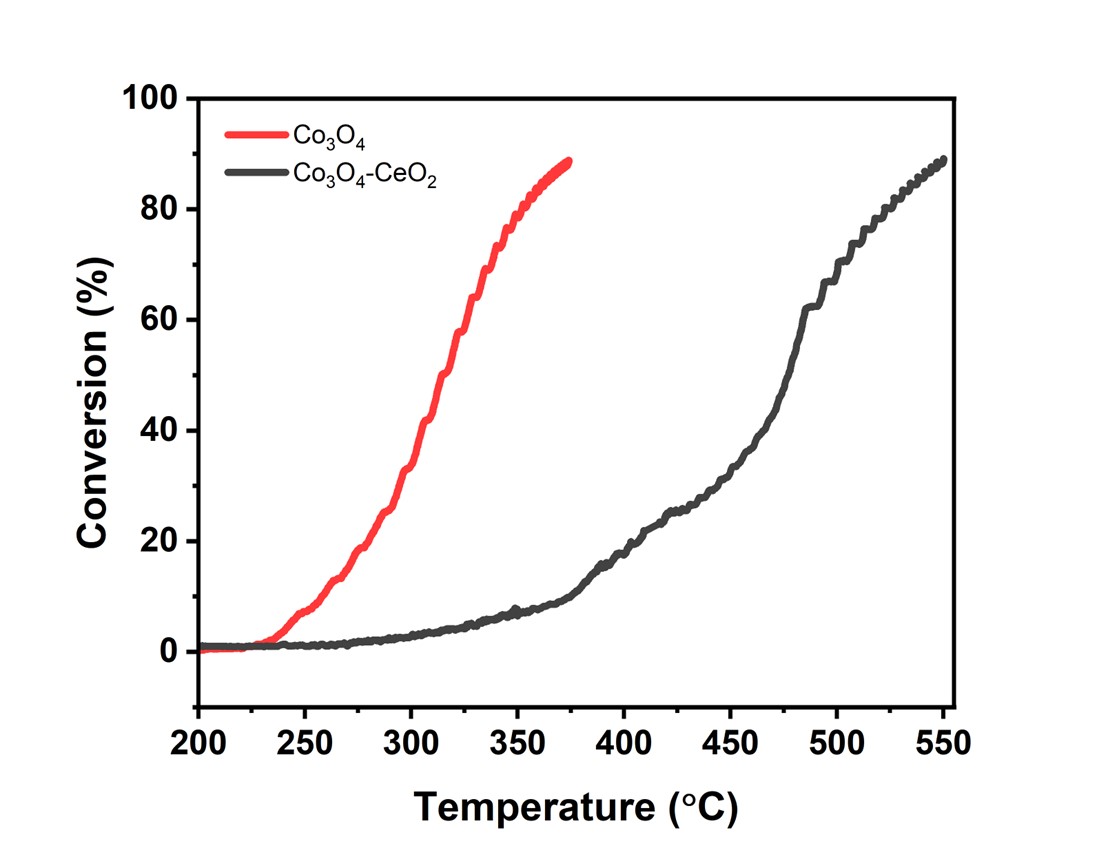
Both bulk Co3O4 and 1:5 Co3O4-CeO2 mixture were synthesized using the co-precipitation method. Catalytic performances of catalysts are presented in Figure 1. Co3O4 catalysts exhibited superior activity than mixed metal oxides in the whole temperature range: the onset of methane conversion is observed at a temperature of 225°C, with an approximate conversion of 90% being attained at 370°C. Respectively, for Co3O4-CeO2, the methane conversion started at a temperature of 325°C and reached a conversion rate of 90% at 550°C.
Figure 1. CH4 conversion (%) over bulk Co3O4 and Co3O4-CeO2. (1vol.%-CH4 in the air) (18000 1/h (NTP))
Implication
This study aims to explore catalytic conversion methods for reducing non-CO2 greenhouse gas emissions, particularly methane (CH4) at low concentrations and proposes using transition metal oxides as catalysts to convert CH4. Additionally, the study intends to unravel conversion mechanisms on different transition metals and investigate the effects of synthesis methods on catalytic functions of the different catalysts. These findings hold promise for the development of efficient technology aimed at mitigating non-CO2 greenhouse gas emissions, particularly from agricultural and waste sectors, which could aid in limiting global warming.
Acknowledgment
The work is part of the project “Removing non-CO2 greenhouse gas emissions to support ambitious climate transitions (REPAIR)” (Project number: 101069905) funded by the European Commission via the European Climate, Infrastructure and Environment Executive Agency (CINEA) within the Horizon Europe framework.
References
- Summary for Policymakers, in Global Warming of 1.5°C: IPCC Special Report on Impacts of Global Warming of 1.5°C above Pre-industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, Ipcc, Editor. 2022, Cambridge University Press: Cambridge. p. 1-24.
- Stolaroff, J.K., et al., Review of Methane Mitigation Technologies with Application to Rapid Release of Methane from the Arctic. Environmental Science & Technology, 2012. 46(12): p. 6455-6469.
- Lan, X., K.W. Thoning, and E.J. Dlugokencky: Trends in globally-averaged CH4, N2O, and SF6 determined from NOAA Global Monitoring Laboratory measurements. Version 2024-01, https://doi.org/10.15138/P8XG-AA10.
- Sirigina, D.S.S.S., A. Goel, and S.M. Nazir, Process concepts and analysis for co-removing methane and carbon dioxide from the atmosphere. Scientific Reports, 2023. 13(1): p. 17290
- Lucía M. Toscani, Pablo A. Curyk, M. Genoveva Zimicz, Emilia B. Halac, Martín. Saleta, Diego G. Lamas, Susana A. Larrondo, Methane catalytic combustion over CeO2-ZrO2-Sc2O3 mixed oxides, Applied Catalysis A: General, 2019, 587, https://doi.org/10.1016/j.apcata.2019.117235.
- Huang, X., Li, J., Wang, J. et al. Catalytic combustion of methane over a highly active and stable NiO/CeO2 catalyst. Front. Chem. Sci. Eng. 14, 534–545 (2020). https://doi.org/10.1007/s11705-019-1821-4