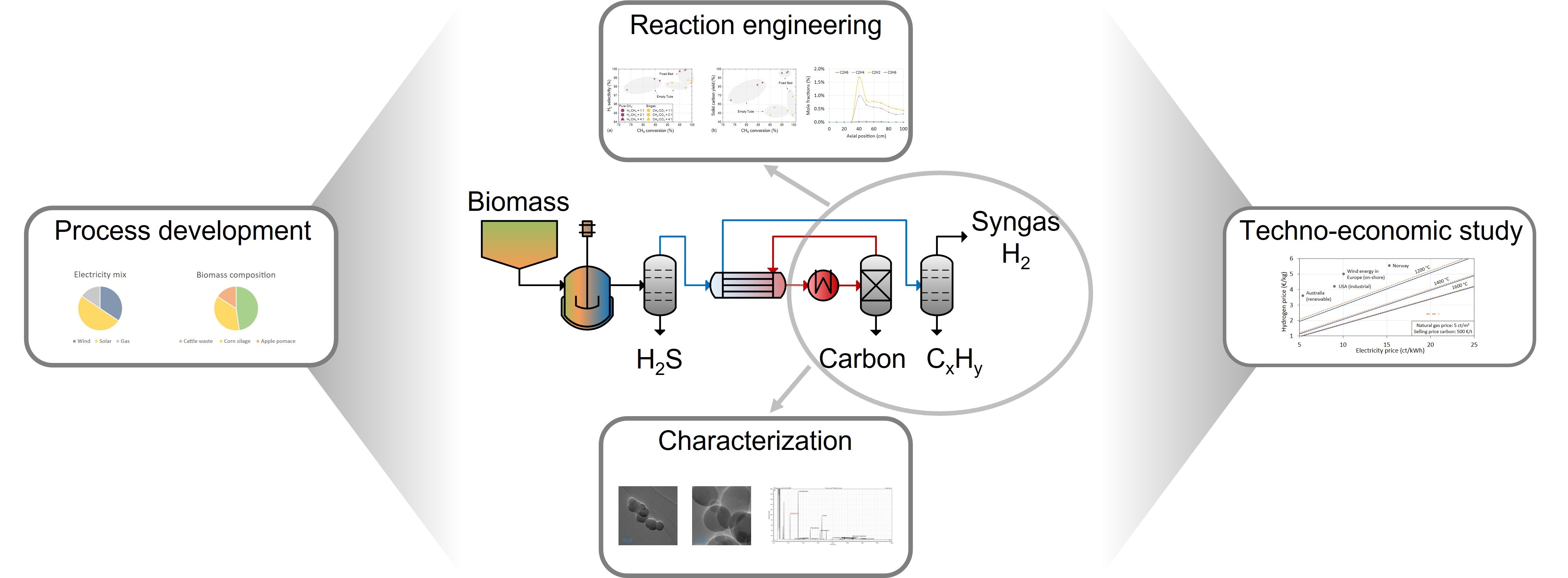
1. Introduction
The pyrolysis of methane (CH4) allows to produce hydrogen (H2) without direct carbon dioxide (CO2) emissions [1,2]. If the CH4 originates from fermentation of biomass, the GHG emissions are even negative as the CH4/CO2 mixture in the biogas is converted to syngas [3]. This study presents a comprehensive exploration of the development of a competitive process for hydrogen or syngas production using methane sources such as natural gas, flaring gas, or biogas. Experimental data on gas phase species concentrations and resulting carbon, obtained at a high-temperature setup, facilitate process optimization for each methane source from both techno-economic and ecological perspectives.
2. Methods
Pyrolysis experiments were conducted in a high-temperature setup [2,3] operated between 1000 °C and 1600 °C and with residence times between 1 s and 7 s at atmospheric pressure. Mass spectrometry (end-of-pipe) and gas chromatography (spatially resolved data along the reactor) were used to analyze gaseous products. Solid carbonaceous species formed during the reaction were characterized with regard to morphology, surface, structure, and particle size (TEM, BET, DLS, XDS, Raman) for potential industrial applications. Species boiling at high temperatures, for instance polyaromatic hydrocarbons (PAH), were characterized via gas chromatography. Aspen Plus was employed for process development, considering techno-economic aspects through the calculation of both mass and energy balances.
3. Results and discussion
First, the thermal pyrolysis as well as the thermo-catalytic pyrolysis with carbonaceous materials of methane, synthetic natural gas (SNG), and biogas is investigated in a high-temperature setup, whereby the influence of process parameters – temperature, residence time, pressure, and H2 dilution – is clarified for each feed gas stream. Herein, industrially relevant CH4 conversions (>80%), CO2 conversions (>97%), and H2 selectivities (>97%) can be achieved at temperatures above 1400 °C and residence times above 5 s while simultaneously fixing over 90% (for CH4 and SNG) or 70% (for biogas), respectively, of the elemental carbon as solid product. The presence of a carbonaceous fixed bed accelerates heterogeneous reactions during carbon deposition, which increases both CH4 and CO2 conversion and suppresses the formation of undesired by-products such as C2 species or benzene.
In addition to the aforementioned parameter studies, mechanistic aspects must be considered. For all feeds, a strong inhibiting effect of the dilution gas H2 can be observed, which can reduce the CH4 conversion by up to 50% compared to an inert gas dilution, but also prevents by-product formation in the gas phase. The formation of propylene is particularly critical, as it does not act as an intermediate for solid formation but can rather be regarded as a dead-end in the reaction system and thus impedes the complete decomposition to carbon and H2. Furthermore, for SNG as feed, a positive influence of the additional hydrocarbons in the feed on CH4 conversion and H2 selectivity can be observed. Most likely this is due to the faster decomposition of higher hydrocarbons compared to methane; radicals formed from these higher hydrocarbons may then accelerate subsequent dehydrogenation steps.
For biogas as feed, further reactions such as dry reforming, the Boudouard reaction, and the (reverse) water-gas shift reaction must be considered. Since syngas (H₂/CO mixture) is the primary product when biogas is used as feed, various biogas compositions and their corresponding biomass feedstocks were studied. A target H₂:CO ratio of 2:1 in the product gas stream was chosen since this is commonly used in syntheses processes relying on syngas. Using a CH₄:CO₂ feed gas ratio of 1:1, for instance as typically obtained from the fermentation of cattle slurry or corn silage, led to the desired target syngas ratio. The mechanistic investigations were supported by spatially resolved measurements of the gas phase composition in a flow reactor, which allowed conclusions to be drawn about the formation of any intermediates and by-products.
As in terms of mass the solid carbon is the main product of the reaction, a comprehensive characterization is necessary in order to evaluate the industrial feasibility of the pyrolysis process. The morphology, surface, structure and particle size of the carbon produced are thoroughly analyzed using fundamental methods such as transmission electron microscopy (TEM), N2-physisorption, dynamic light scattering (DLS) or Raman spectroscopy. In addition, potential utilization options for the produced carbon are investigated. For instance, one option studied in more detail is carbon as reinforcing additive in rubber materials.
In order to utilize the resulting carbon, the formation of high-boiling products like PAHs that adsorb onto it and that are partially regulated due to their toxicity must be examined more closely. These products, which are liquid under standard conditions, are first separated from the solid using suitable solvents and then separated into aliphatic and aromatic compounds using solid phase extraction. In this way, a qualitative analysis as well as a quantitative analysis can be carried out using offline gas chromatography. This approach allows a precise qualitative identification of different species as well as a determination of their concentration in relation to the total product gas formed. PAHs in particular form the main part of the high-boiling species; the absolute quantity and the size of the molecules, however, depend very much on the process conditions. For this reason, a comprehensive parameter study is carried out to work out the influence of temperature, residence time, and H2 dilution on the PAH composition. Herein, especially reaction temperatures between 1200 and 1300 °C and residence times between 3 and 5 s seem to promote the formation of PAH, which can bind around 1% of the produced H2. However, higher reaction temperatures suppress the formation of PAHs.
In combination with the results of the carbon and PAH characterization, the kinetic tests are the basis for a techno-economic study. Herein, all data were fed into Aspen Plus simulations in order to design industrially feasible processes for hydrogen and syngas production from natural gas or biogas. Results from energy and mass balances were used to evaluate overall energy consumption, operating costs, and capital costs as functions of both process parameters and external parameters. This study determined a hydrogen price of approximately 2 €/kg and a syngas price between 1 and 1.5 €/kg. A graphical overview of the conducted work is provided in Figure 1.
4. Conclusions
Our work comprises a process development for the production of climate-friendly hydrogen or syngas from pyrolysis processes with variable feedstock. Based on experimental parameter studies on various methane sources – pure CH4, natural gas, flaring gas, biogas – as well as a comprehensive characterization of the resulting carbon and any high-boiling products, industrially viable overall process concepts were developed. Herein, H2 prices of approx. 2 €/kg and syngas prices of 1 to 1.5 €/kg can be realized. Compared to conventional processes, the pyrolysis of methane or natural gas and flaring gas is quite competitive, whereas syngas production from biogas calls for further process optimization. Notably, the competitiveness for each process is highly dependent on the carbon price. Suitable applications, e.g. carbon use in rubber or tire production, can result in significantly higher carbon prices compared to the values assumed in this study, which would substantially increase the economic appeal of syngas production from biogas.
References
[1] N. Sánchez-Bastardo, R. Schlögl, H. Ruland, Methane pyrolysis for zero-emission hydrogen production: A potential bridge technology from fossil fuels to a renewable and sustainable hydrogen economy, Ind. Eng. Chem. Res. 60 (2021) 11855–11881.
[2] P. Lott, M.B. Mokashi, H. Müller, D.J. Heitlinger, S. Lichtenberg, A.B. Shirsath, C. Janzer, S. Tischer, L. Maier, O. Deutschmann, Hydrogen production and carbon capture by gas phase methane pyrolysis: A feasibility study, ChemSusChem 16 (2023) e202300300.
[3] A. Çelik, I. Ben Othman, H. Müller, P. Lott, O. Deutschmann, Pyrolysis of biogas for carbon capture and carbon dioxide-free production of hydrogen, React. Chem. Eng. 9 (2024) 108-118.