In this study, process variables namely temperature, molar ratio of CO
2:H
2, and Gas-hourly space velocity (GHSV) were varied to (i) obtain optimal condition and (ii) apparent activation energy (E
a). Catalysts including 100Ni (15wt.%), 75Ni25Fe (15wt.%), 100Rh (3wt.%) and 100Ru (3wt.%) were examined. Therefore, a series of reactions have been carried out by varying (i) reaction temperature (523 - 773 K), (ii) partial pressure of CO
2 (at 1:4, 1:8 and 1:24 molar ratio of CO
2:H
2), (iii) contact time (at a GHSV of 7643 h
-1, 15287 h
-1 and 22930 h
-1). Furthermore, temperature dependency of the catalysts was manifested by determining E
a using power law model.
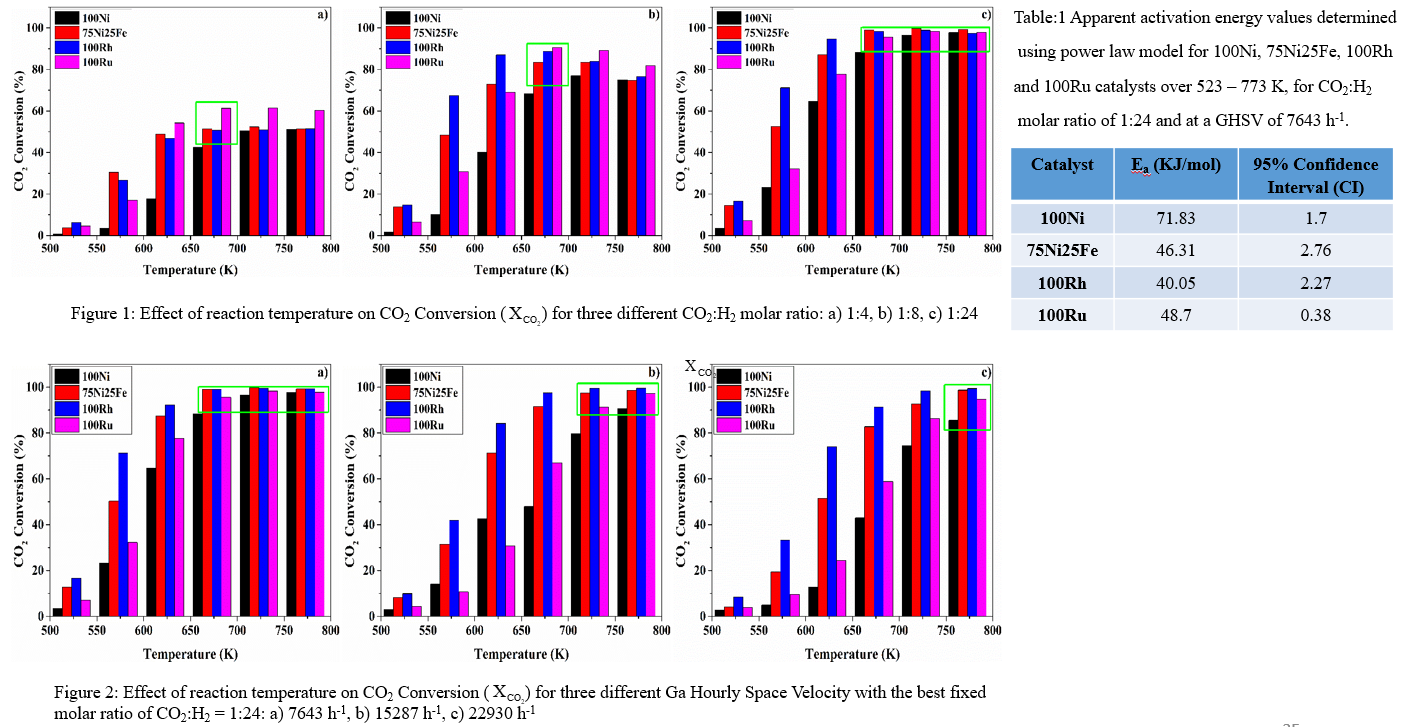
Figure 1 reveals that, the Ru catalyst outperforms others over 623 – 773 K, indicating its superior ability to activate CO2 towards methane without excess hydrogen. However, increasing hydrogen partial pressure by increasing the molar ratio, the performance of Ni-Fe and Rh approaches similar to Ru. At excess of hydrogen (CO2:H2 = 1:24) all the catalysts exhibit similar CO2 conversion rate over 673 – 773 K. Nevertheless, at low temperature Rh and Ni-Fe catalysts were superior to Ru, suggests high hydrogen partial pressure’s significance in activating CO2 over Rh and Ni-Fe. Also, at molar ratio of CO2:H2 =1:24 and temperature of 673 K at a GHSV of 7643 h-1 is obtained as optimal condition. Moreover, effect of GHSV (at CO2:H2 = 1:24) was studied in Figure 2, revealing CO2 conversion decreased for all catalysts with increasing GHSV. However, Rh and Ni-Fe catalysts remained superior to Ru and Ni irrespective of GHSV in presence of excess hydrogen. Finally, using power law, Ea was calculated over 523 – 773 K and at pCO2 of 0.04 atm. and pH2 of 0.96 atm. for all the catalysts indicating the trend in kinetic activity as 100Ni < 75Ni25Fe ~ 100Ru < 100Rh.